Synthesis process of gingerol derivative
A technology of derivatives and gingerol, which is applied in the field of synthetic technology of gingerol derivatives, can solve the problems that synthesis research and process development are still in their infancy, and achieve the advantages of convenient industrial production, high yield, and inhibition of by-products generated effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
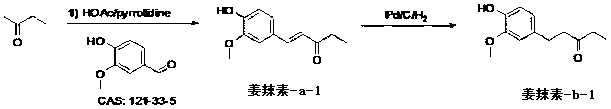
[0024] Embodiment 1, ( E )-1-(4-hydroxy-3-methoxyphenyl)pent-1-en-3-one ( gingerol-a-1 )Synthesis:
[0025] Into a 5 liter glass reactor was charged 120 grams of glacial acetic acid and 140 grams of tetrahydropyrrole. The flask is equipped with an electric stirring rod, a thermometer and a dropping funnel. Soak the flask in ice water, and when the temperature of the solution in the bottle drops to gingerol-a-1 Crude. oily crude gingerol-a-1 Dissolve in 10 liters of warmed petroleum ether (30°C) containing 100 g of activated carbon. The mixture was stirred for 1 hour, filtered while hot, and the filtrate was cooled to 5°C to obtain pale yellow needle-like crystals. Yield: 350 g. Melting point: 93 degrees.
Embodiment 2
[0026] Embodiment 2, 1-(4-hydroxy-3-methoxyphenyl)pentan-3-one ( Gingerol-b-1 )Synthesis:
[0027] In a 5 liter flask add 100 g gingerol-a-1 , 2 liters of methanol and 2 grams of palladium on carbon catalyst (containing 10% palladium). The reaction mixture was replaced with hydrogen 3 times, then stirred under hydrogen atmosphere for 3 hours. The mixture was filtered and the filtrate was concentrated to give the crude product Gingerol-b-1 . Pure Gingerol-b-1 Obtained by silica gel column chromatography. per 100g crude product Gingerol-b-1 The oil was dispersed with 200g of silica gel (100mesh), and then eluted with ethyl acetate / petroleum ether (2%~10%) to obtain a pure oil Gingerol-b-1. Gingerol-b-1 It can be recrystallized into needle crystals by petroleum ether. Yield: 79 grams. Melting point: 37~38 degrees.
Embodiment 3
[0028] Embodiment 3, ( E )-1-(4-hydroxy-3-methoxyphenyl)hex-1-en-3-one ( gingerol-a-2 ) and 1-(4-hydroxy-3-methoxyphenyl)hexan-3-one ( Gingerol-b-2) Synthesis:
[0029] gingerol-a-2 and gingerol-b-2 It is synthesized by a method similar to that of Examples 1 and 2. gingerol-a-2 Melting point: 89 degrees; gingerol-b-2 Melting point: 44 degrees.
PUM
| Property | Measurement | Unit |
|---|---|---|
| carbon number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com