Method for measuring trace triptorelin
A triptorelin and trace technology, applied in the field of trace triptorelin determination, can solve problems such as injury to testing personnel, unsuitability for clinical and scientific research, and expensive kits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
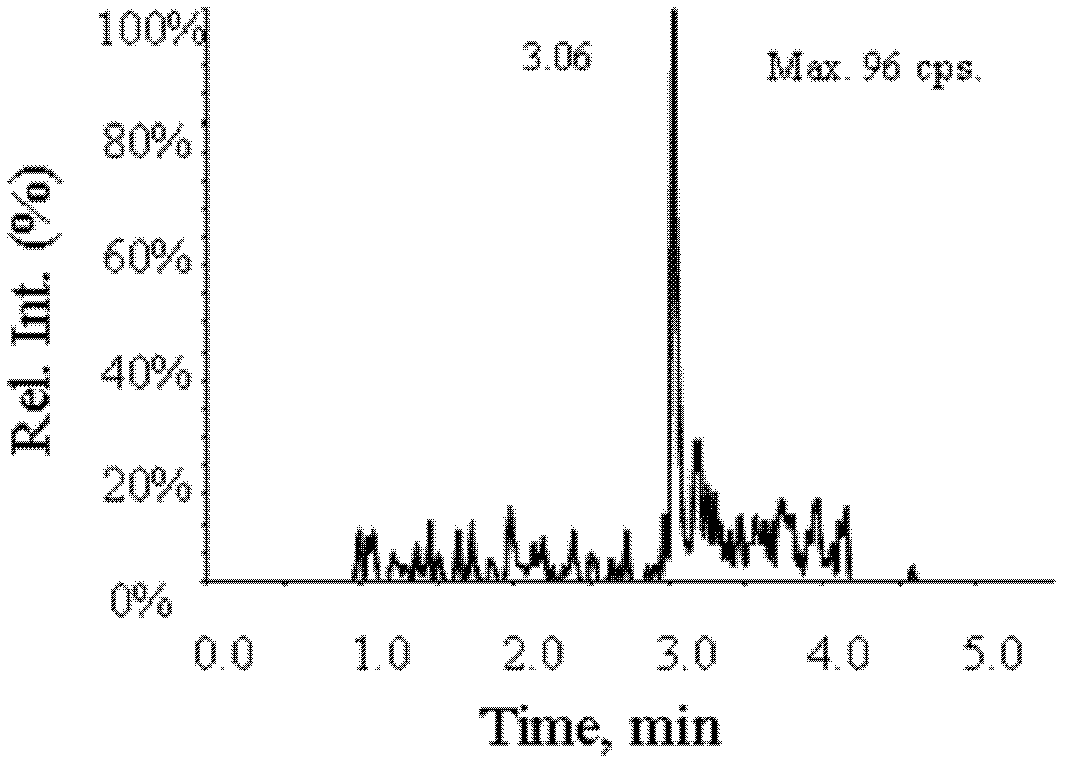
[0013] Example 1 The mobile phase is 0.02% acetic acid aqueous solution-methanol to detect triptorelin
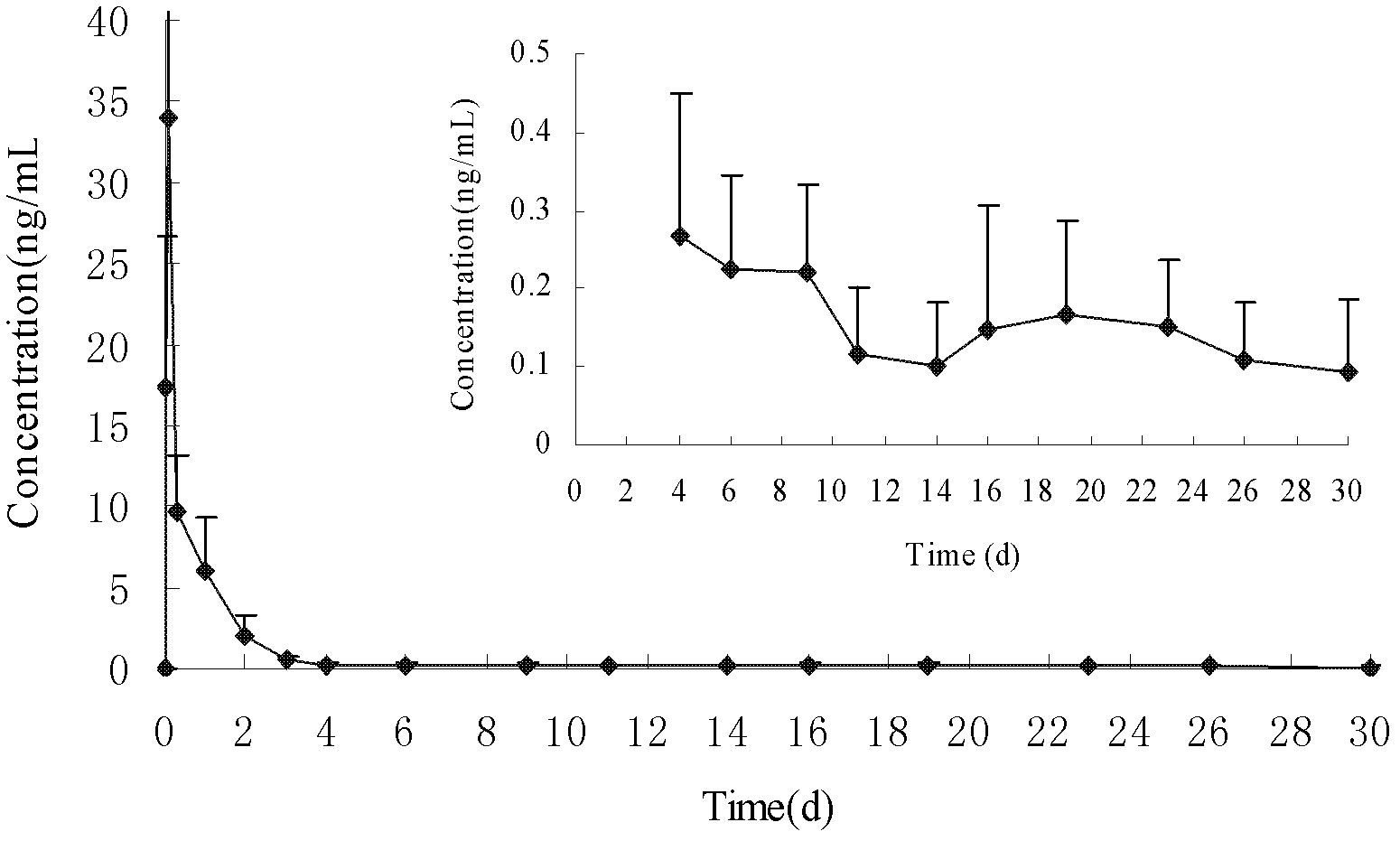
[0014] Preparation of Standard Series Solution Samples Accurately weigh 25 mg of triptorelin standard, put it in a 25 ml volumetric flask, add methanol: water: formic acid (80: 20: 0.04) solution to dissolve, and dilute to the mark to prepare 1 mg / ml stock solution. Take an appropriate amount of triptorelin stock solution and dilute with methanol: water: formic acid (60:40:0.08) to 0.005, 0.01, 0.03, 0.1, 0.3, 1.0, 3.0, 10.0 ng / ml.
[0015] LC Conditions Column: Venusil MP-C18 column (2.1 mm x 50 mm, 3 μm, Agela). Mobile phase: A: 0.02% acetic acid aqueous solution, B: methanol; flow rate: 0.6mL / min, column temperature: 40°C, injection volume: 10μl. Agilent1290 high performance liquid chromatography system, including binary infusion pump, automatic sampler, column thermostat. The gradient conditions are as follows:
[0016] Time(min)
A
B
...
Embodiment 2
[0020] Example 2 The mobile phase is 0.02% acetic acid aqueous solution-methanol to detect triptorelin
[0021] The preparation of standard series solution sample is the same as embodiment 1
[0022] LC Conditions Column: Venusil MP-C18 column (2.1 mm x 50 mm, 3 μm, Agela). Mobile phase: A: 0.02% acetic acid aqueous solution, B: methanol; flow rate: 0.6mL / min, column temperature: 40°C, injection volume: 10μl. Agilent1290 high performance liquid chromatography system, including binary infusion pump, automatic sampler, column thermostat. The gradient conditions are as follows:
[0023] Time(min)
A
B
0
90
10
1
90
10
2.3
40
60
2.7
40
60
2.71
2
98
4
2
98
4.01
90
10
5.5
90
10
[0024] Mass spectrometry conditions: QTRAP5500 mass spectrometer, equipped with ion spray ionization source, ...
Embodiment 3
[0027] Example 3 The mobile phase is 0.05% acetic acid aqueous solution-methanol detection triptorelin
[0028] The preparation of standard series solution sample is the same as embodiment 1
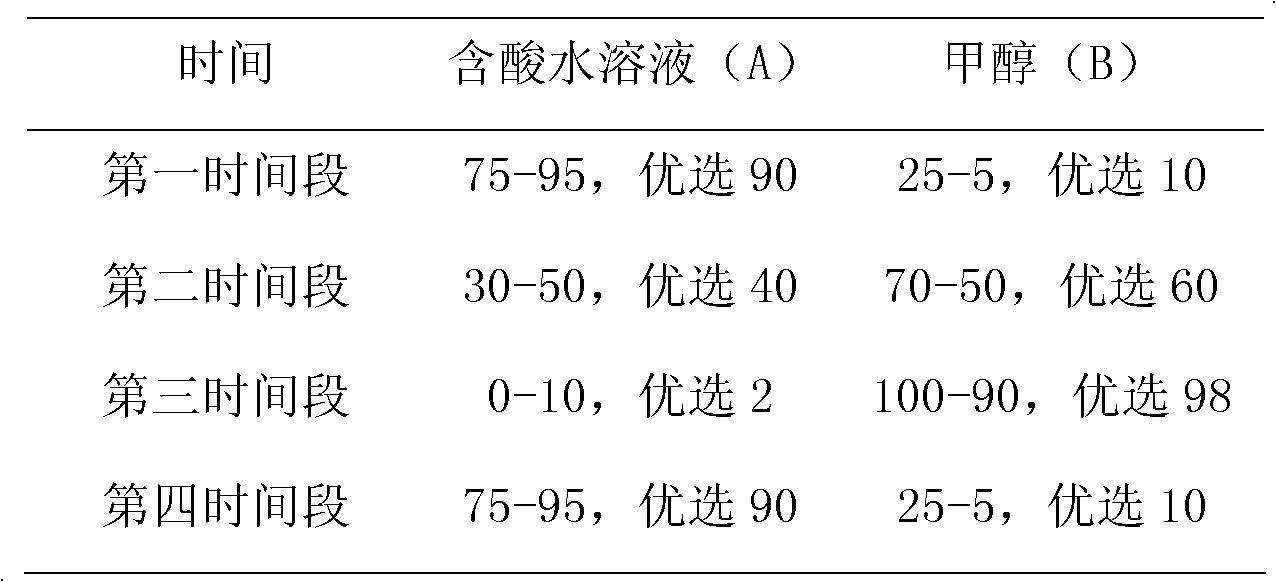
[0029] LC Conditions Column: Venusil MP-C18 column (2.1 mm x 50 mm, 3 μm, Agela). Mobile phase: A: 0.05% acetic acid aqueous solution, B: methanol; flow rate: 0.4 mL / min, column temperature: 30°C, injection volume: 10 μl. Agilent 1290 high performance liquid chromatography system, including binary infusion pump, automatic sampler, column thermostat. The gradient conditions are as follows:
[0030] Time(min)
A
B
0
90
10
1
90
10
4.0
50
50
5.0
50
50
5.1
5
95
6.5
5
95
7.0
90
10
9.0
90
10
[0031] Mass spectrometry conditions: QTRAP5500 mass spectrometer, equipped with ion spray ionization sourc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| collision energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com