Hemostasis bandaid and preparation method thereof
A first-aid bandage and bandage technology, applied in tourniquets, bandages, textiles, etc., can solve the problems of increased treatment costs for patients, poor anti-leakage effect of gauze, and impaired vision, and meet the requirements of industrialized scale-up production and good absorption. The effect of preventing liquid leakage and reducing the cost of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
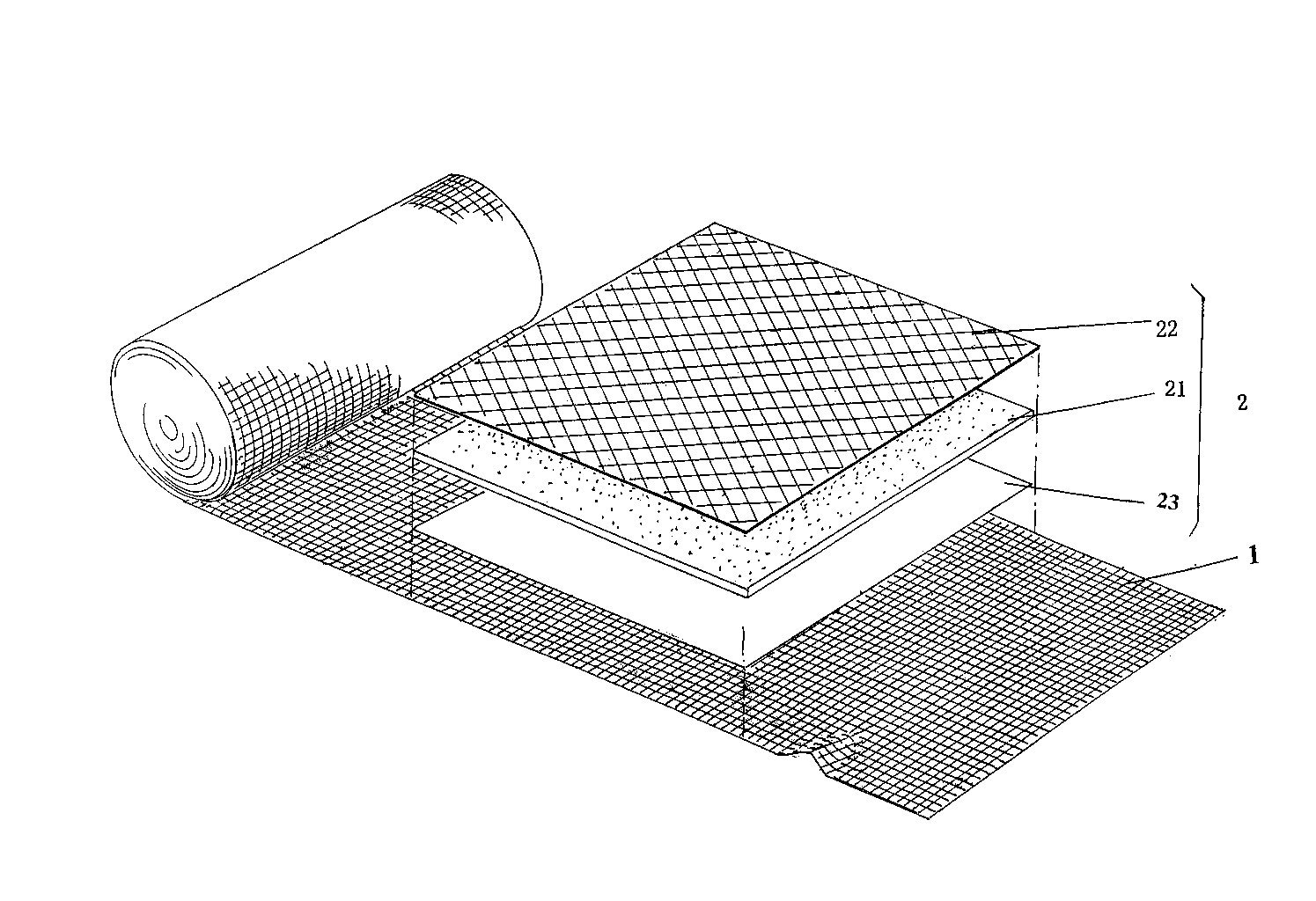
[0020] preparation figure 1 The steps of the hemostatic bandage with the structure shown are as follows:
[0021] A) To prepare the wound treatment pad, the anti-adhesion film 22 with a grammage of 15g / ㎡ is heated and compounded by a thermal compounding machine, and compounded to a non-woven alginate fiber with a grammage of 160g at a heating compounding temperature of 110°C The cloth 21 faces one side of the patient's wound in the use state, and the polyurethane breathable film 23 with a grammage of 2 g / ㎡ is heated and composited to the other side of the alginate fiber non-woven fabric 21 at a temperature of 105 ° C. side, and then cut into a cube with a side length of 10 cm by a cutting machine to obtain a wound treatment pad 2. The anti-adhesion film described in this step is preferably used by the British Smith & Nephew company in the Chinese market before this application is proposed. The grade is CM-type mesh isolation film or the mesh isolation film sold in China ...
Embodiment 2
[0024] Only change the gram weight of the anti-adhesion film 22 in step A) to 20g / ㎡, change the heating composite temperature of the anti-adhesion film 22 and the alginate fiber non-woven fabric 21 to 115°C, and the alginate fiber non-woven fabric 21 The gram weight was changed to 200g / ㎡, and the alginate fiber non-woven fabric 21 was produced by Changshu Mintai Medical Dressing Co., Ltd., Jiangsu Province, China and sold on the market before this application was filed. The brand is MT-Ⅱ-A biological fiber For non-woven fabrics, change the heating composite temperature of the polyurethane breathable film 23 and the alginate fiber non-woven fabric 21 to 115°C, change the weight of the polyurethane breathable film 23 to 3g / ㎡, and change the side length of the wound treatment pad 2 after cutting Change it to 15cm; change the ironing temperature in step B) to 125°C, change the transition distance to 10cm, change the length of the bandage body 1 to 360cm, and change the width to 1...
Embodiment 3
[0026] Only change the grammage of the anti-adhesion film 22 in step A) to 17g / ㎡, change the heating composite temperature of the anti-adhesion film 22 and the alginic acid fiber non-woven fabric 21 to 120°C, and the alginic acid fiber non-woven fabric 21 Change the gram weight to 120g / ㎡, and change the heating composite temperature of the polyurethane breathable film 23 and the alginate fiber non-woven fabric 21 to 110°C, and change the gram weight of the polyurethane breathable film 23 to 5g / ㎡. Change the side length of pad 2 to 5cm; change the ironing temperature in step B) to 125°C, change the transition distance to 8cm, change the length of bandage body 1 to 305cm, and change the width to 5cm , and the rest are the same as those described in Example 1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com