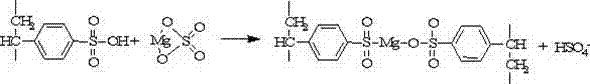
Method for preparing catalyst for catalytic synthesis of dialkyl succinate
A catalyst and sulfate technology are applied in the field of catalyst preparation for catalytic synthesis of dialkyl succinate, can solve problems such as difficulty in reaction, and achieve the effects of easy product separation, good catalyst stability and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 100 g of macroporous styrene-based strong-acid cation exchange resin into a reactor equipped with a stirrer, a reflux condenser, a thermometer, and a dropping funnel, add 150 ml of concentrated HCl, and add concentrated H 2 SO 4 30ml, FeCl 3 ·6H 2 O 3g, slowly drop in 30mlH from the dropping funnel 2 o 2 Solution, reacted at 60°C for 3h. Filter the reaction product, wash it with acid and then wash it with water to neutrality, and dry it in vacuum at 80°C to obtain the chlorinated heat-resistant resin;
[0035] Put 100% succinic acid and n-butanol with a molar ratio of 1:3.4 into a reactor equipped with a water separator, agitator, thermometer and condenser, and add 3% of the total reactant mass obtained in the previous step The chlorinated heat-resistant resin catalyst was used, the reaction temperature was 115°C, and the reaction time was 60 minutes to obtain the esterified product. The esterification rate is listed in Table 1.
Embodiment 2
[0039] Add 100 g of macroporous styrene-based strong-acid cation exchange resin into a reactor equipped with a stirrer, a reflux condenser, a thermometer, and a dropping funnel, add 150 ml of concentrated HCl, and add concentrated H 2 SO 4 50ml, FeCl 3 ·6H 2 O 10g, slowly add 60mlH from the dropping funnel 2 o 2 Solution, reacted at 90°C for 6h. Filter the reaction product, wash it with acid and then wash it with water to neutrality, and dry it in vacuum at 80°C to obtain the chlorinated heat-resistant resin;
[0040] Put 80% succinic acid aqueous solution and n-butanol with a molar ratio of 1:3.4 into a reactor with a water separator, agitator, a thermometer and a condenser tube, and add 3% of the total reactant mass prepared in the previous step. The obtained chlorinated heat-resistant resin catalyst was filtered at a reaction temperature of 115°C and a reaction time of 60 minutes. The used chlorinated heat-resistant resin was washed several times with absolute ethanol...
Embodiment 3
[0044] Add 100 g of macroporous styrene-based strong-acid cation exchange resin into a reactor equipped with a stirrer, reflux condenser, thermometer, and dropping funnel, add 120 ml of concentrated HCl, and add 120 ml of concentrated HCl. 2 SO 4 30ml, FeCl 3 ·6H 2 O 5g, slowly add 45mlH from the dropping funnel 2 o 2 Solution, reacted at 80°C for 4h. Filter the reaction product, wash it with acid and then wash it with water to neutrality, and dry it in vacuum at 80°C to obtain the chlorinated heat-resistant resin;
[0045] Immerse the chlorinated heat-resistant resin prepared in the previous step in 1% CaSO 4 After immersing in the solution for 1 hour at room temperature, evaporate to dryness with a rotary evaporator at 80°C, take it out and dry it in vacuum at 80°C to obtain a chlorinated heat-resistant calcium sulfate-loaded modified resin catalyst;
[0046] Put 70% succinic acid aqueous solution and n-butanol with a molar ratio of 1:3.4 into a reactor with a water s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com