Catalyst for methanation of carbon dioxide
A methanation catalyst, carbon dioxide technology, applied to the field of carbon monoxide methanation catalyst, improved carbon dioxide, carbon dioxide hydrogenation to obtain methane or removal of trace carbon oxides from hydrogen, can solve the problem of insufficient reaction space velocity, low air yield, Alkylation activity is not enough to achieve the effect of good stable structure and thermal stability, easy to scale up production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
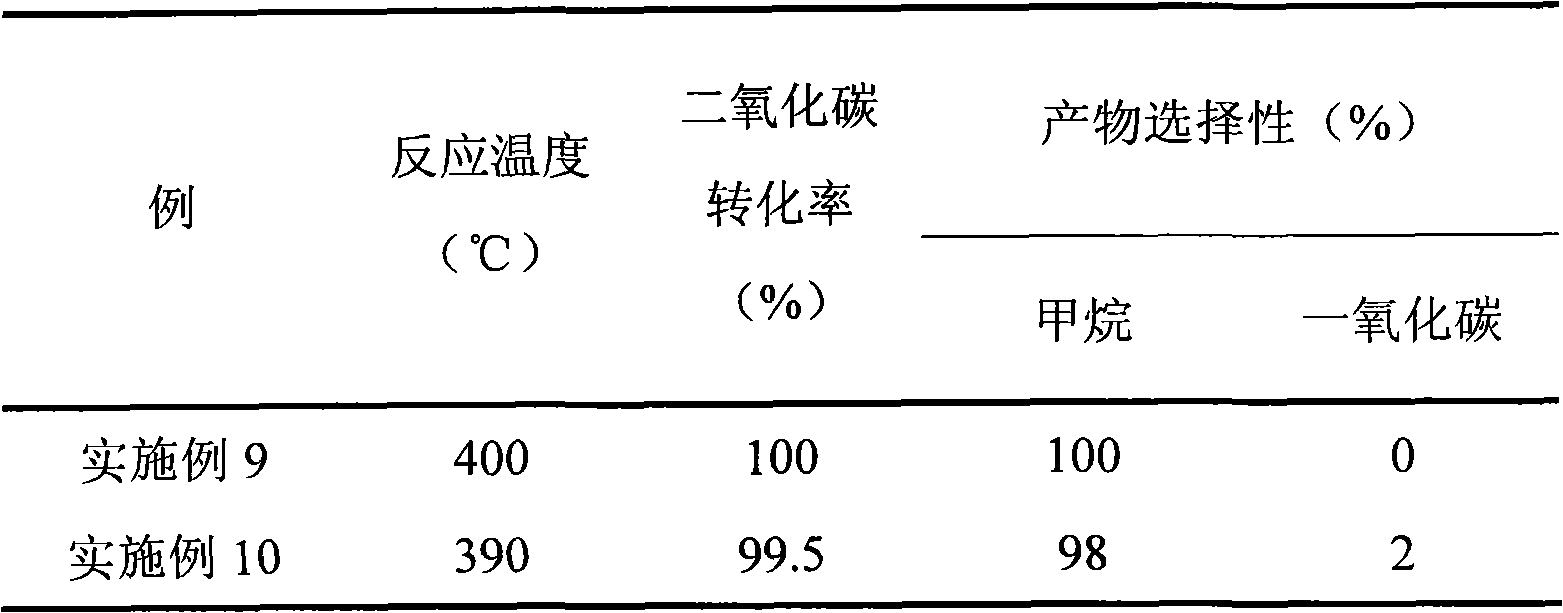
Examples
Embodiment 1
[0042] 10.8 g of lanthanum nitrate hexahydrate and 8.8 g of ferric nitrate nonahydrate were dissolved in 100 ml of distilled water (referred to as solution I), and 6.1 g of sodium hydroxide was dissolved in 100 ml of distilled water (referred to as solution II). Take another 100ml of distilled water in a beaker, heat up to 50°C, add solution I and II dropwise while stirring, and keep the pH of the solution at 7-8. After the dropwise addition, continue stirring for 5 hours, filter, and wash with deionized water until sodium-free ion. After the precipitate was dried at 110-120 for 5 hours, it was calcined at 650°C for 4 hours to obtain a dark red carrier (denoted as L 1 ). The carrier was analyzed by XRD to be a perovskite structure with a specific surface area of 78 square meters per gram.
Embodiment 2
[0044] 10.8 g of lanthanum nitrate hexahydrate and 7.3 g of cobalt nitrate hexahydrate were dissolved in 100 ml of distilled water (referred to as solution I), and 8.5 g of sodium hydroxide was dissolved in 100 ml of distilled water (referred to as solution II). Take another 100ml of distilled water in a beaker, raise the temperature to 70°C, add solutions I and II dropwise while stirring, and keep the pH of the solution at 9-10. After the dropwise addition, continue to stir for 5 hours, filter and wash with deionized water until sodium-free ion. After the precipitate was dried at 110-120 for 5 hours, it was roasted at 700°C for 4 hours to obtain the carrier (denoted as L 2 ). The carrier was analyzed by XRD as a perovskite structure with a specific surface area of 65 square meters per gram.
Embodiment 3
[0046] 22 grams of zinc acetate dihydrate, 75 grams of aluminum nitrate nonahydrate and 36 grams of urea were mixed and ground evenly, dried at 180°C for 12 hours, and roasted at 550°C for 5 hours to obtain a light yellow powder carrier (referred to as L 3 ), which is a spinel structure by XRD analysis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com