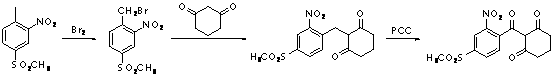
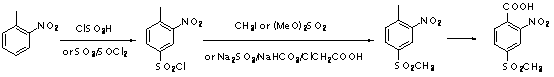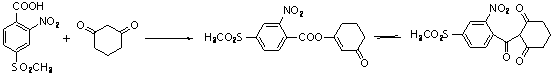Preparation method of 2-(2-nitryl-4-methyl sulfuryl-benzoyl) cyclohexane-1,3-diketone
A benzoyl and methylsulfonyl technology is applied in the field of preparation of 2-(2-nitro-4-methylsulfonyl-benzoyl)cyclohexane-1,3-dione, and can solve the reaction steps Many problems, lack of competitiveness, immaturity, etc., to achieve the effect of simple operation and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Synthesis of 2-(2-nitro-4-methylsulfonyl-benzylhydroxy)cyclohexane-1,3-dione (MST01)
[0039] In a 2L four-necked flask, add 2-nitro-4-methylsulfonylbenzaldehyde (114.5g / 0.5mol), 1,3-cyclohexanedione (67.2g / 0.6mol) and 400ml toluene in sequence, stir and mix Control the temperature of the reaction system at 10~15℃, add N, N-dimethylbenzylamine (94.5g / 0.7mol) dropwise, after the dropwise addition, continue the heat preservation reaction for 1.5h, stop the reaction, use dilute sulfuric acid (wt 20 %) Adjust the system to neutral to obtain a mixed solution of intermediate MST01, which can be directly used in the next reaction.
[0040] (2) Synthesis of 2-(2-nitro-4-methylsulfonyl-benzoyl)cyclohexane-1,3-dione (MST)
[0041] Control the temperature of the MST01 mixture at 40~45℃, add 30% hydrogen peroxide (62.33g / 0.55mol), stir and mix well, add 120ml dilute sulfuric acid (wt 20%) dropwise, after the dropwise addition, continue to keep the temperature for 2h, stop heating Th...
Embodiment 2
[0044] (1) Synthesis of 2-(2-nitro-4-methylsulfonyl-benzylhydroxy)cyclohexane-1,3-dione (MST01)
[0045] In a 2L four-necked flask, add 2-nitro-4-methylsulfonylbenzaldehyde (114.5g / 0.5mol), 1,3-cyclohexanedione (67.2g / 0.6mol) and 400ml tetrahydrofuran in sequence, and stir to mix. Evenly, control the temperature of the reaction system at 35-40℃, add 400ml sodium hydroxide aqueous solution (wt 5%) dropwise, after the dropwise addition, continue to keep the reaction for 40 minutes, stop the reaction, adjust the system to neutral with dilute sulfuric acid (wt 20%) , The mixed solution of intermediate MST01 can be directly used in the next reaction.
[0046] (2) Synthesis of 2-(2-nitro-4-methylsulfonyl-benzoyl)cyclohexane-1,3-dione (MST)
[0047] Control the temperature of the MST01 mixture at 55~60℃, add peroxyacetic acid (41.5g / 0.55mol) dropwise, stir and mix, after the dropwise addition, continue to keep the reaction for 1h, stop heating, and cool the reaction solution to 0 with an i...
Embodiment 3
[0050] (1) Synthesis of 2-(2-nitro-4-methylsulfonyl-benzylhydroxy)cyclohexane-1,3-dione (MST01)
[0051] In a 2L four-necked flask, add 2-nitro-4-methylsulfonylbenzaldehyde (114.5g / 0.5mol), 1,3-cyclohexanedione (67.2g / 0.6mol) and 400ml sulfolane, stir and mix Control the temperature of the reaction system at 10-15°C, add 300ml of sodium carbonate aqueous solution (wt 15%) dropwise, after the dropwise addition, continue the heat preservation reaction for 2h, stop the reaction, adjust the system to neutral with dilute sulfuric acid (wt 20%), The mixed solution of intermediate MST01 can be directly used in the next reaction.
[0052] (2) Synthesis of 2-(2-nitro-4-methylsulfonyl-benzoyl)cyclohexane-1,3-dione (MST)
[0053] At room temperature, add ceric ammonium nitrate (164.4g / 0.3mol) to the mixture of MST01, stir and mix, add 50ml dilute sulfuric acid (wt 20%) dropwise, after the dropwise addition, continue to react at room temperature for 3h, stop heating, the reaction solution Use ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com