Reaction product of stannyl derivative of naphthalimide and rylene
A stannyl and rylene technology, applied in the field of compositions and compounds, can solve the problems of difficult to separate dibrominated mixtures, difficult to obtain metallizing reagents, difficult to mass-produce, etc., and achieves improved solid state filling. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0159] Additional specific embodiments are provided below without limitation.
[0160] PART I.
Embodiment A-1
[0161] Embodiment A-1 prepares compound 3,4
[0162] Scheme 1. Preparation of stannyl NDI derivatives.
[0163]
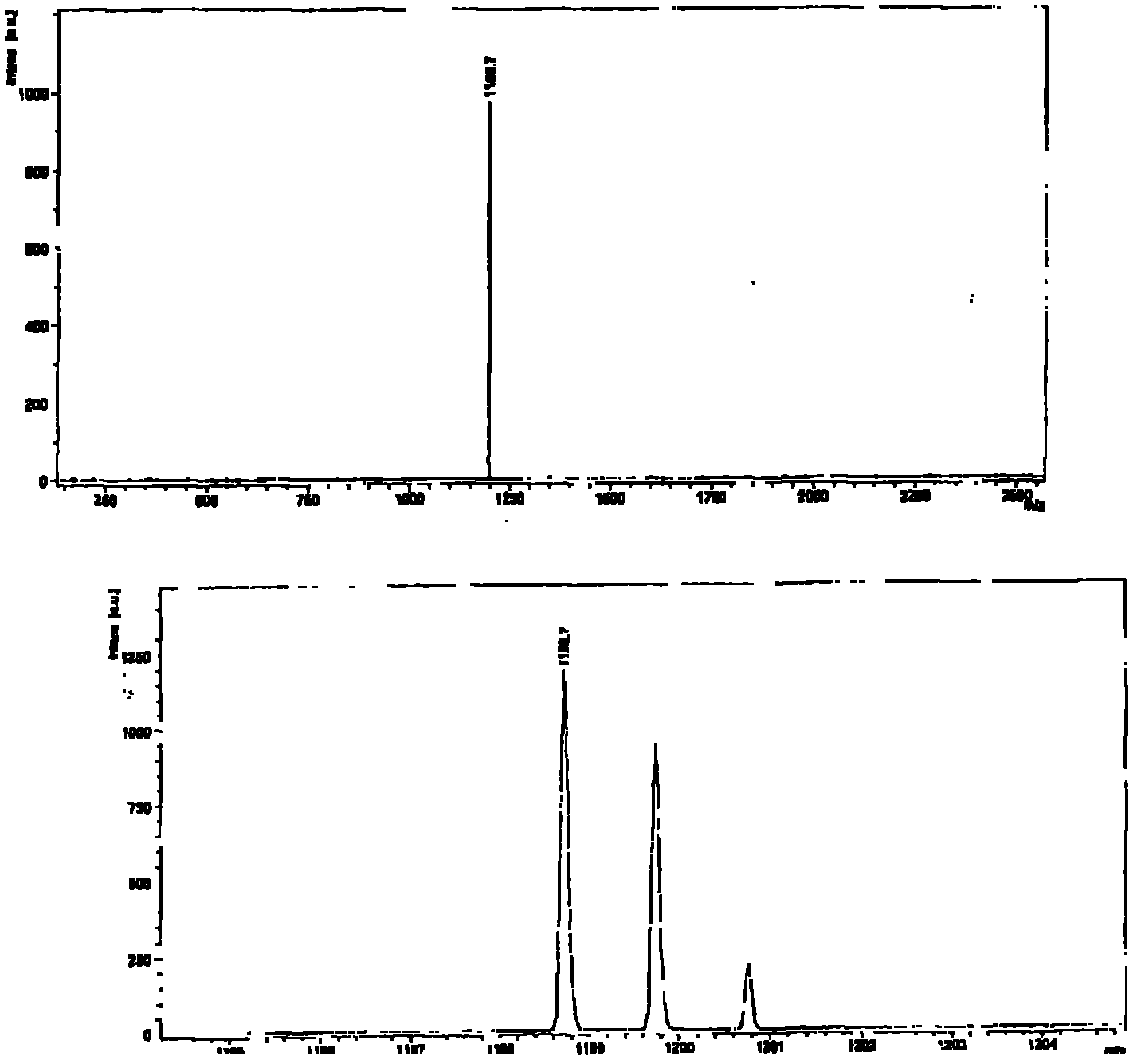
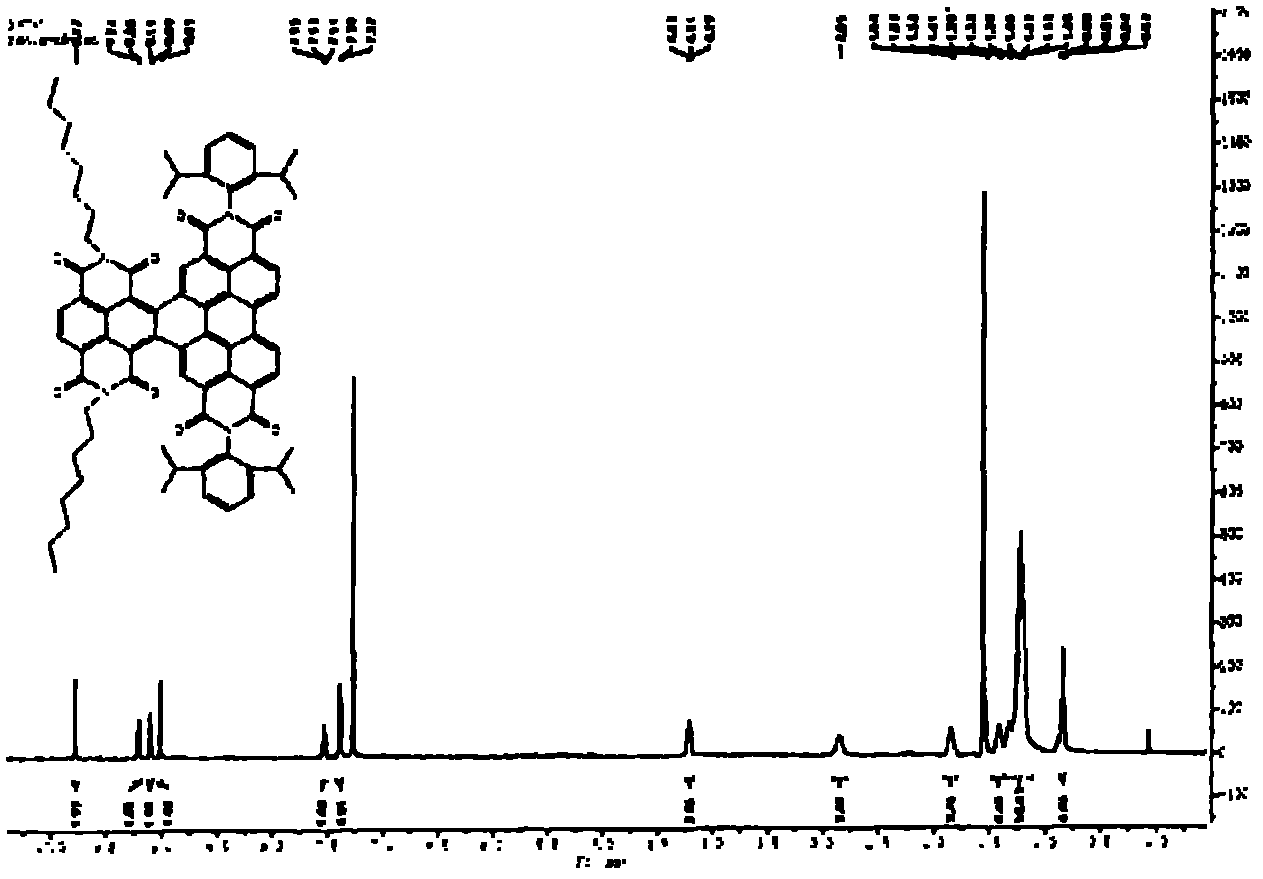
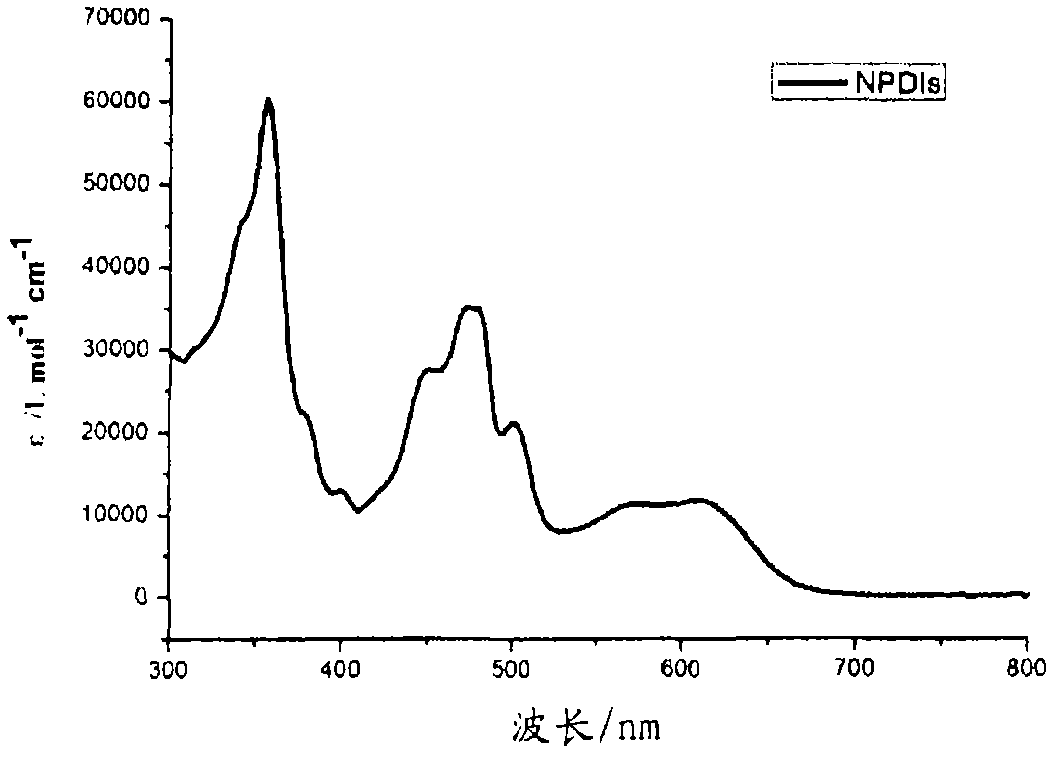
[0164] N,N'-bis(n-hexyl)-2-tris-(n-butyl)stannylnaphthalene-1,4,5,8-bis(dicarboximide), 3, and N,N' -bis(n-hexyl)-2,6-bis(tri(n-butyl)stannyl)naphthalene-1,4,5,8-bis(dicarboximide), 4, according to scheme 1 with moderate The yield is obtained. A mixture of suitable mono- or dibrominated derivatives, 1 or 2, and hexabutylditin (1 equivalent per brominated substituent) in toluene on Pd 2 dba 3 (0.05 equiv per bromide) and P(o-tol) 3 (0.2 equivalent per bromide) was heated. The reaction products were purified by silica gel chromatography and recrystallization from methanol to provide mono- and stannyl derivatives as long yellow needles; these compounds were analyzed by NMR spectroscopy, mass spectroscopy, elemental analysis and, in In the case of compound 4, the crystal X-ray structure was characterized.
[0165] Under the same conditions, in comparison, mon...
Embodiment A-2
[0167] Embodiment A-2 replaces preparation method; The synthesis of 5 and 6
[0168] Scheme 2. Preparation of stannyl NDI derivatives from commercially available NDA.
[0169]
[0170] Compounds 3 and 4 have different chromatographic properties (3: Rf = 0.3 on silica, eluting with 1:1 dichloromethane / hexane; 4: Rf = 0.3 on silica, using 1:10 dichloromethane / hexane elution), it is recommended to use a mixture of mono- and dibromo species for this reaction, which comes from the bromination and imylation of NDA and is only purified in the final stage. It is even possible to carry out these transformations without isolation of the mono- and di-functionalized intermediates, to obtain isolated yields of the mono- and stannyl derivatives of approximately 20% and 5%, respectively (e.g., when using 1eq.DBI said brominating agent).
[0171] The relative yields can be adjusted according to the brominating agent, about 10% yields were obtained for using 2.1 eq. of DBI mono- and di-s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com