Star silsesquioxane-grafted acrylic ester-sulfonated styrene segmented copolymer and preparation method thereof
A technology of silsesquioxane and sulfonated styrene, used in electrochemical generators, fuel cells, electrical components, etc. The effect of hydrophilicity and superior thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
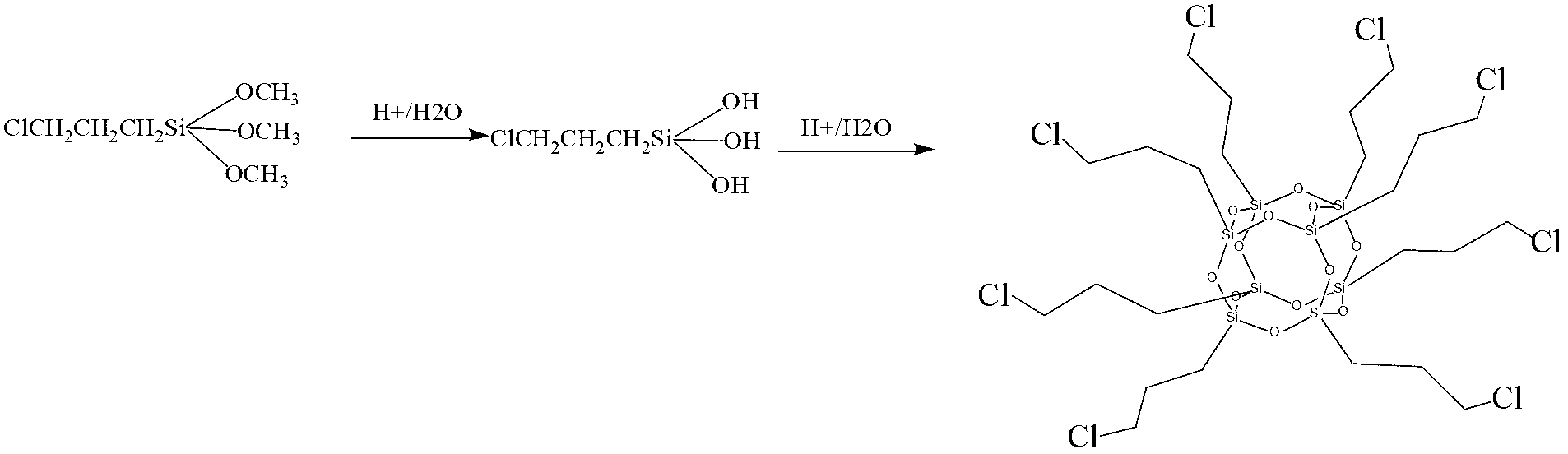
[0027] (1) Step 1: Add 600ml of anhydrous methanol, 24ml of concentrated hydrochloric acid, and 30ml of γ-chloropropyltrimethoxysilane to a 1000ml three-necked flask in sequence, and continuously stir the reaction at 40°C for 5 days to obtain a white solid. Rinse several times, and dry in a vacuum oven at 50°C for 48 hours. Synthetic process such as figure 1 shown;
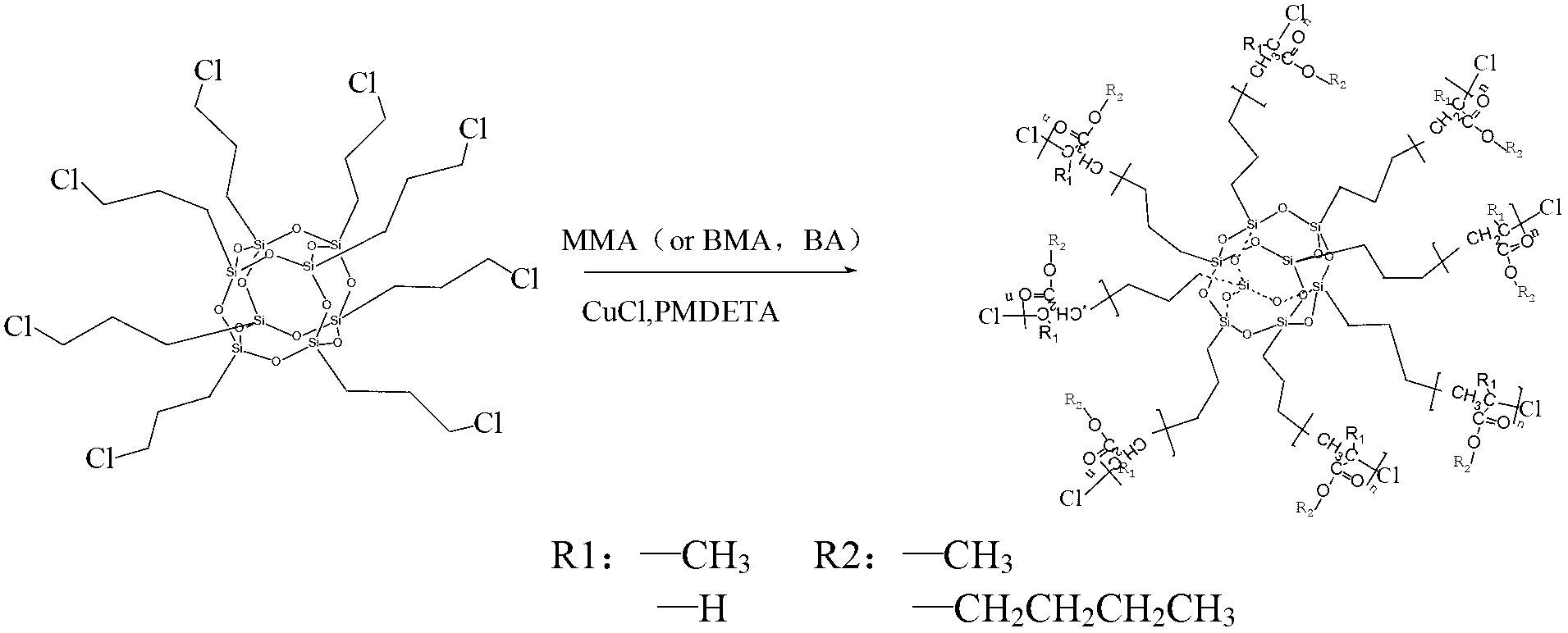
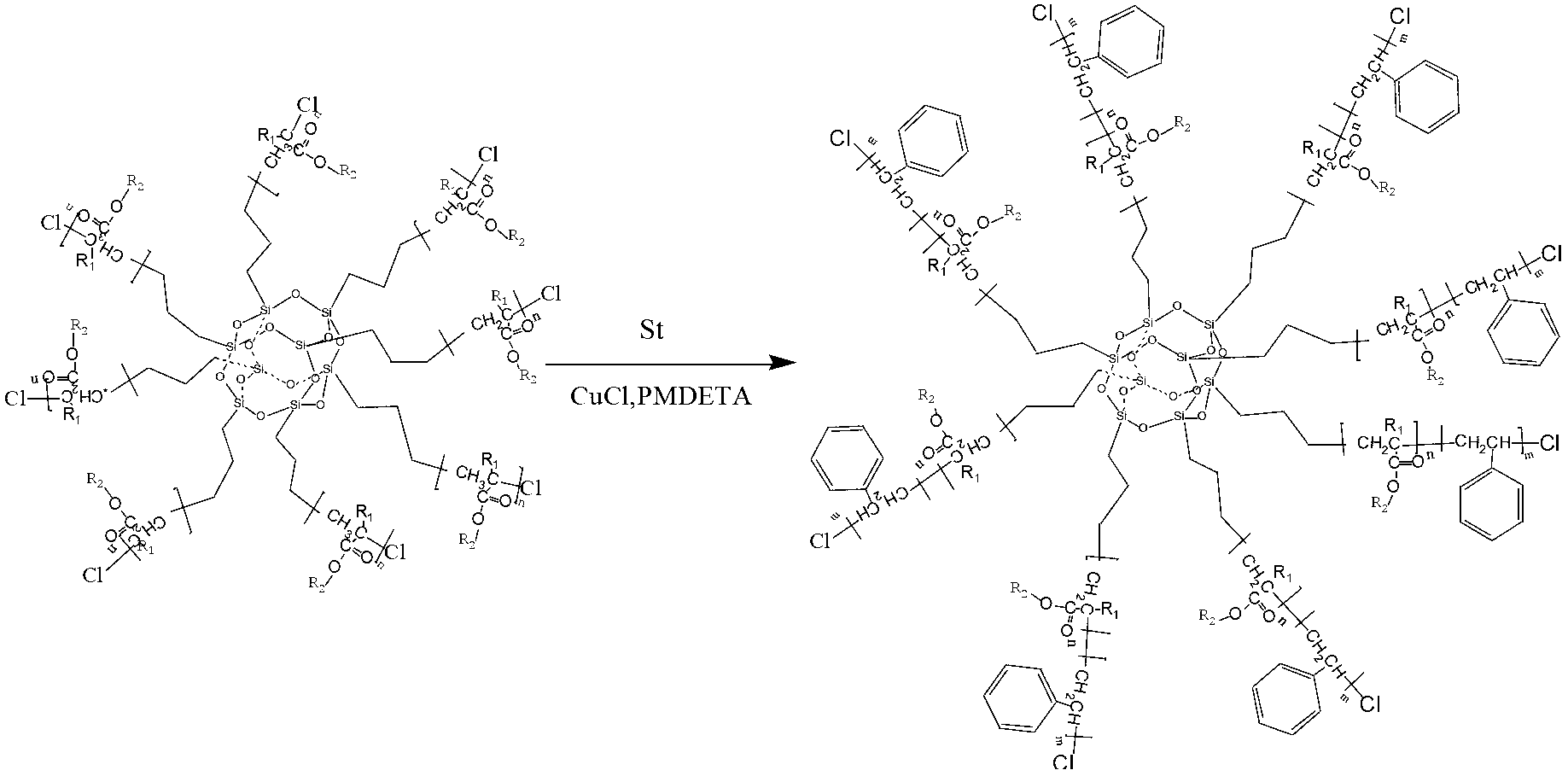
[0028] Step 2: Mix the measured octachloropropylsilsesquioxane (0.2g), pentamethyldivinyltriamine (0.15ml), cuprous chloride (0.015g), methyl methacrylate (20ml ) and toluene (20ml) were respectively added in a 100ml three-neck flask equipped with a condenser tube and a magnetic stirrer, stirred under nitrogen protection, the temperature was raised to 70°C, and then gradually increased to 110°C, and reacted to The viscosity of the system increased significantly. Dissolve the product in the container with a large amount of tetrahydrofuran to obtain a polymer solution. Pass the solution through a neutral alumina c...
Embodiment 2
[0043] (1) Step 1: Add 600ml of anhydrous methanol, 24ml of concentrated hydrochloric acid, and 30ml of γ-chloropropyltrimethoxysilane to a 1000ml three-necked flask in sequence, and continuously stir the reaction at 40°C for 5 days to obtain a white solid. Rinse several times, and dry in a vacuum oven at 50°C for 48 hours. Synthetic process such as figure 1 shown;
[0044] Step 2: Mix the measured octachloropropylsilsesquioxane (0.4g), pentamethyldivinyltriamine (0.30ml), cuprous chloride (0.030g), methyl methacrylate (40ml ) and toluene (40ml) were respectively added in a 250ml three-neck flask equipped with a condenser tube and a magnetic stirrer, stirred under nitrogen protection, the temperature was raised to 70°C, and then gradually increased to 110°C, and reacted to The viscosity of the system increased significantly. Dissolve the product in the container with a large amount of tetrahydrofuran to obtain a polymer solution. Pass the solution through a neutral alumina c...
Embodiment 3
[0059] (1) Step 1: Add 600ml of anhydrous methanol, 24ml of concentrated hydrochloric acid, and 30ml of γ-chloropropyltrimethoxysilane to a 1000ml three-necked flask in sequence, and continuously stir the reaction at 40°C for 5 days to obtain a white solid. Rinse several times, and dry in a vacuum oven at 50°C for 48 hours. Synthetic process such as figure 1 shown;
[0060] Step 2: Mix the measured octachloropropylsilsesquioxane (0.2g), pentamethyldivinyltriamine (0.15ml), cuprous chloride (0.015g), methyl methacrylate (20ml ) and toluene (20ml) were respectively added in a 100ml three-neck flask equipped with a condenser tube and a magnetic stirrer, stirred under nitrogen protection, the temperature was raised to 70°C, and then gradually increased to 110°C, and reacted to The viscosity of the system increased significantly. Dissolve the product in the container with a large amount of tetrahydrofuran to obtain a polymer solution. Pass the solution through a neutral alumina c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com