Biodegradable unimolecular multi-support-arm polymer as well as preparation method f and application thereof
A polymer and single-molecule technology, applied in the field of medicine, can solve problems such as poor water solubility and stability, limited effective treatment of tumors, and large toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
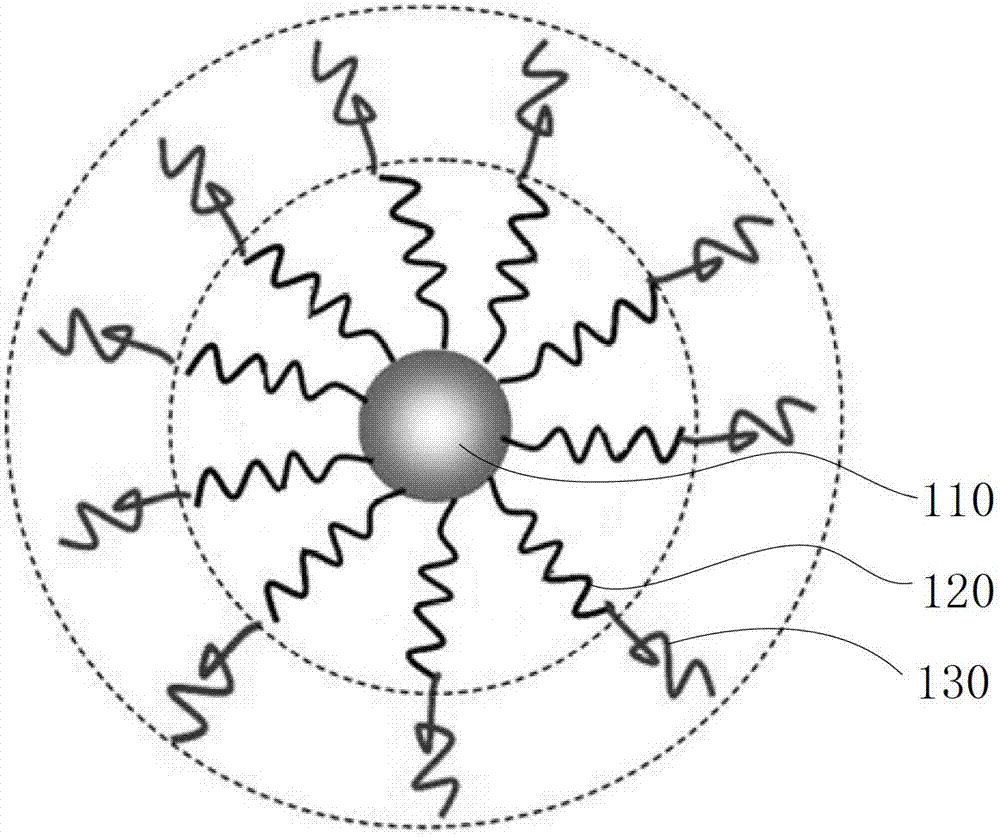
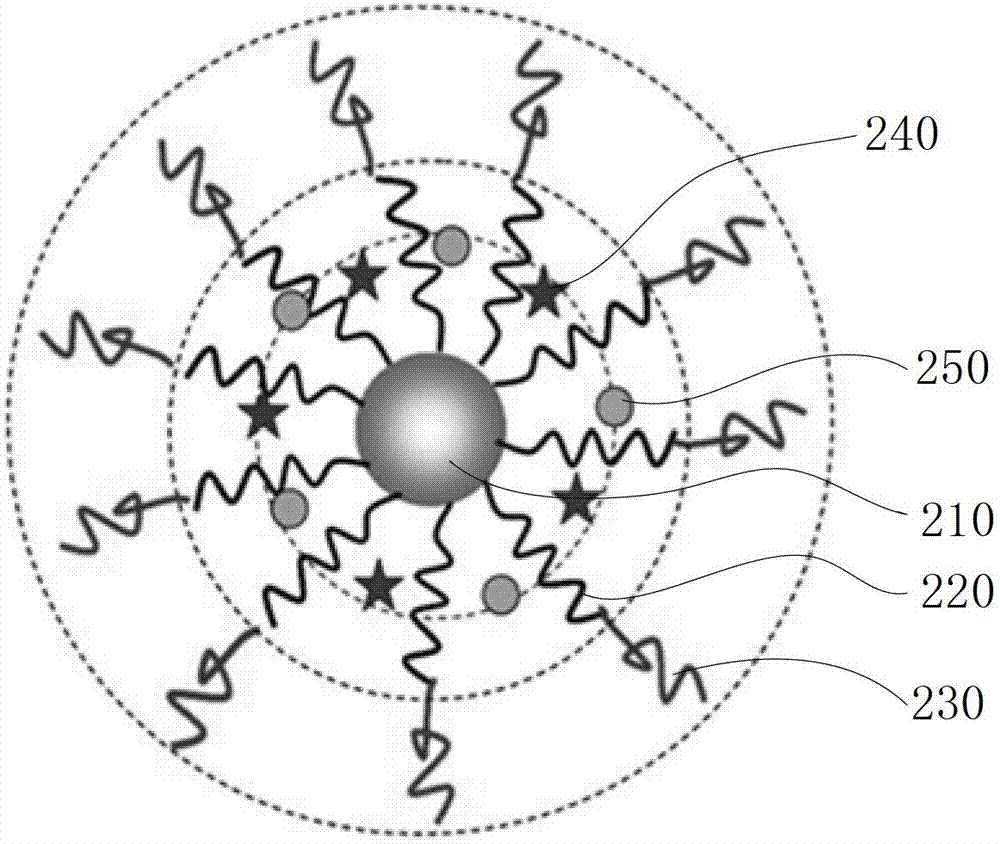
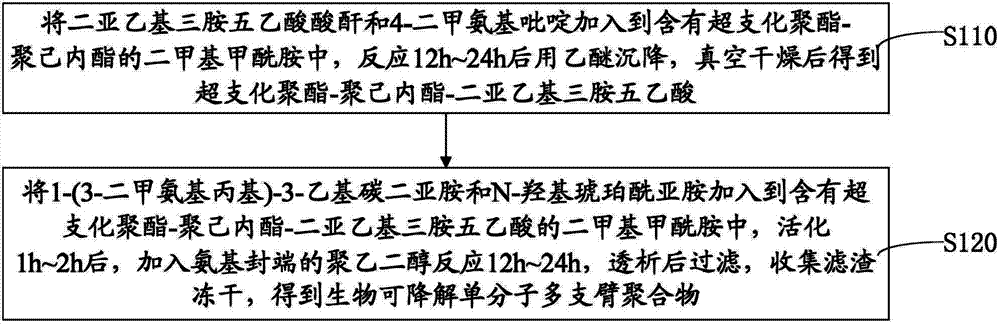
[0061] Please refer to image 3 and Figure 5 , the preparation method of the above-mentioned biodegradable single-molecule multi-arm polymer, comprising the following steps:
[0062] Step S110, adding diethylenetriaminepentaacetic anhydride and 4-dimethylaminopyridine into dimethylformamide containing hyperbranched polyester-polycaprolactone, reacting for 12h to 24h, settling with ether, vacuum After drying, hyperbranched polyester-polycaprolactone-diethylenetriaminepentaacetic acid is obtained.
[0063] The molar ratio of the hydroxyl unit of hyperbranched polyester-polycaprolactone, diethylenetriaminepentaacetic anhydride and 4-dimethylaminopyridine is 1:10-20:0.2.
[0064] Hyperbranched polyester-polycaprolactone is prepared by the following steps:
[0065] Under vacuum conditions, react hyperbranched polyester, caprolactone and stannous octoate at a temperature of 125°C to 135°C for 8h to 12h, and then settle with cold ether to obtain hyperbranched polyester-polycaprol...
Embodiment 1
[0115] 400mg H40, 1500mg caprolactone and 1.5mg stannous octoate (Sn(Oct) 2 ) into a completely dry silanized polymerization tube with a magnetic stirring bar. The polymerization tube was purged with nitrogen three times before use, then sealed under vacuum, and stirred in an oil bath at 135°C for 8 h. Then dissolve the reacted mixture in chloroform, then quickly add a sufficient amount of cold ether for precipitation, collect the filter residue after filtration, dry the filter residue at room temperature and vacuum for two days to obtain H40-PCL (the hydroxyl group of H40 and the mole of PCL The ratio is 1:15).
[0116] Add 492.91mg of DTPA and 2mg of 4-dimethylaminopyridine (DMAP) into dimethylformamide (DMF) containing 200mg of H40-PCL. After 12 hours of reaction, settle with ether to obtain H40-PCL-DTPA.
[0117] Add 34.29 mg 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) and 10.32 mg N-hydroxysuccinimide (NHS) to DMF containing 100 mg H40-PCL-DTPA , after activatio...
Embodiment 2
[0121] 200mg H40, 1000mg caprolactone and 1mg stannous octoate (Sn(Oct) 2 ) into a completely dry silanized polymerization tube with a magnetic stirring bar, the polymerization tube was purged with nitrogen three times before use, then sealed under vacuum, and stirred in an oil bath at 125 °C for 12 h. Then dissolve the reacted mixture in chloroform, then quickly add a sufficient amount of cold ether for precipitation, collect the filter residue after filtration, dry the filter residue at room temperature and vacuum for two days to obtain H40-PCL (the hydroxyl group of H40 and the mole of PCL The ratio is 1:20).
[0122] Add 328.6mg of DTPA and 2mg of 4-dimethylaminopyridine (DMAP) into dimethylformamide (DMF) containing 200mg of H40-PCL. After 24 hours of reaction, settle with ether to obtain H40-PCL-DTPA.
[0123] Add 27.5 mg 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) and 8.26 mg N-hydroxysuccinimide (NHS) to DMF containing 100 mg H40-PCL-DTPA, After activation fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com