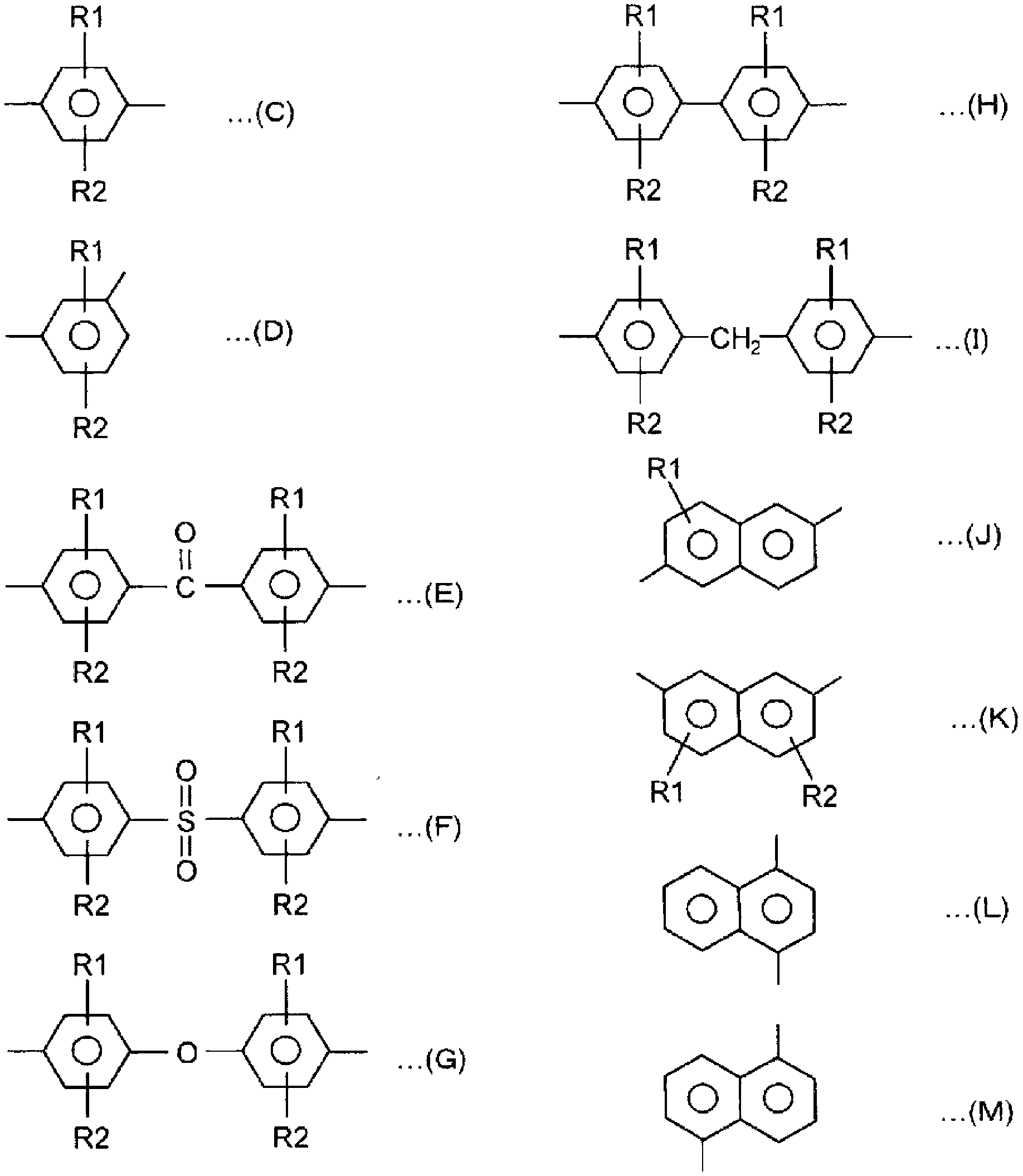
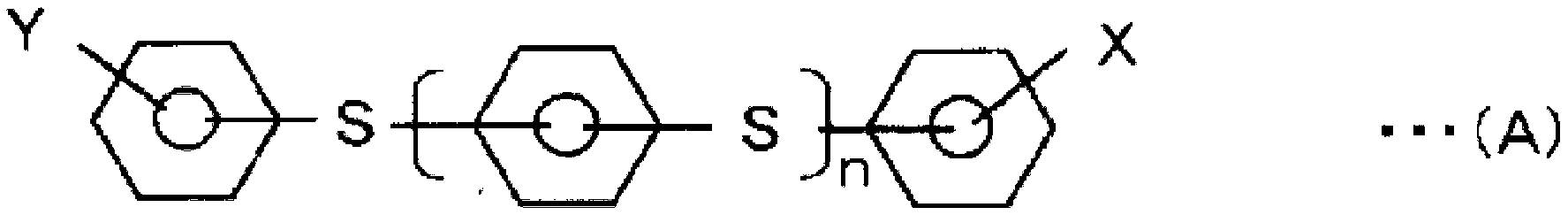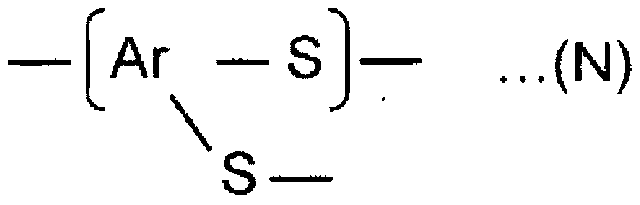Polyarylene sulfide production method and polyarylene sulfide
A technology of polyarylene sulfide and its manufacturing method, which is applied in the field of polyarylene sulfide manufacturing, can solve the problems of increased cost of polyarylene sulfide, and achieve the effect of low gas and high molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0122] The present invention will be specifically described below with reference to examples. These examples are illustrative and not limiting.
[0123]
[0124] The molecular weights of polyarylene sulfide and cyclic polyarylene sulfide were calculated in terms of polystyrene by number average molecular weight (Mn) and weight average molecular weight by gel permeation chromatography (GPC), which is one of size exclusion chromatography (SEC). Molecular weight (Mw). The measurement conditions of GPC are shown below.
[0125] Device: Senshu Science SSC-7100
[0126] Column name: センシューscience GPC3506
[0127] Eluent: 1-Chloronaphthalene
[0128] Detector: Differential Refractive Index Detector
[0129] Column temperature: 210°C
[0130] Pre-heating bath temperature: 250°C
[0131] Pump thermostat temperature: 50°C
[0132] Detector temperature: 210°C
[0133] Flow: 1.0mL / min
[0134] Sample injection volume: 300 μL (slurry form: about 0.2% by weight).
[0135]
[0...
reference example 1
[0188] Reference Example 1 (Preparation of Cyclic Polyarylene Sulfide Composition)
[0189] A stainless steel autoclave equipped with a stirrer was used as a reaction vessel. In the reaction container, 14.03 g (0.120 mol) of a 48% by weight aqueous solution of sodium hydrosulfide, 12.50 g (0.144 mol) of a 48% by weight aqueous solution of sodium hydroxide prepared using 96% sodium hydroxide, and N-methyl-2 - 615.0 g (6.20 mol) of pyrrolidone (NMP) and 18.08 g (0.123 mol) of p-dichlorobenzene (p-DCB). After the inside of the reaction container was sufficiently replaced with nitrogen, it was sealed under a nitrogen atmosphere.
[0190] While stirring at 400 rpm, the temperature was raised from room temperature to 200° C. over about 1 hour. At this stage, the pressure in the reaction vessel was 0.35 MPa in gauge pressure. Then, the temperature was raised from 200°C to 270°C over about 30 minutes. The pressure in the reaction container at this stage was 1.05 MPa in gauge press...
reference example 2
[0196] Reference Example 2 (Preparation of Cyclic Polyarylene Sulfide Composition)
[0197] A stainless steel autoclave equipped with a stirrer was used as a reaction vessel. In the reaction vessel, add 47% sodium hydrosulfide 118g (1 mole), 96% sodium hydroxide 42.3g (1.01 mole), N-methyl-2-pyrrolidone (NMP) 163g (1.66 mole), sodium acetate 24.6g (0.30 mol) and 150 g of ion-exchanged water. The reaction vessel was cooled to 160°C after gradually heating to 240°C over about 3 hours under normal pressure while blowing nitrogen gas and distilling off 212 g of water and 4.0 g (40.4 mmol) of NMP. The amount of hydrogen sulfide scattered was 25 mmol.
[0198] Next, 147 g (1.00 mol) of p-dichlorobenzene (p-DCB) and 129 g (1.30 mol) of NMP were further added, and the reaction container was sealed in nitrogen atmosphere. While stirring at 400 rpm, the temperature was raised from 200°C to 270°C at a rate of 0.6°C / min, and the reaction was continued at 270°C for 140 minutes. Then, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com