Thermosetting crosslinked cycloolefin resin film and manufacturing process therefor
A cycloolefin resin and a manufacturing method technology, which are applied in the directions of printed circuit manufacturing, electric solid state devices, semiconductor/solid state device parts, etc., can solve the problem that the resin does not have mold releasability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~9 and comparative example 1~4
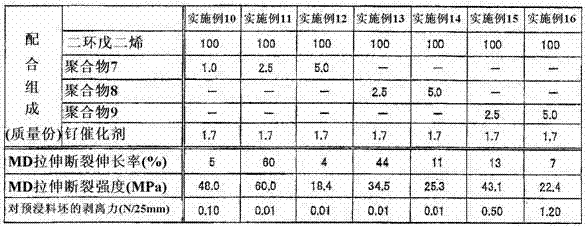
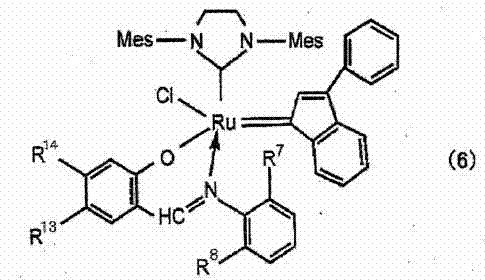
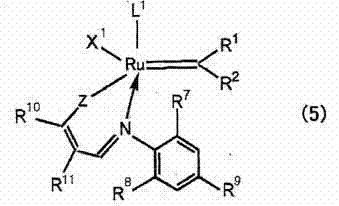
[0123] Prepare the polymers shown in Table 1. A specific polymer containing an alkyl (meth)acrylate unit having an alkyl group having 3 or more carbon atoms having the mass shown in Table 2 and Table 3 was dissolved in dicyclopentadiene to obtain a reaction stock solution. Then, add the ruthenium catalyst with the structure of formula (7) shown in Table 2 and Table 3 to the above reaction stock solution, mix with a line mixer, and then heat Cast film formation was carried out on a carrier film made of polyethylene terephthalate. Then, it heated at 200 degreeC for 5 minutes in nitrogen atmosphere, and obtained the release film. The results are shown in Table 2 and Table 3.
[0124] [chemical 5]
[0125]
[0126] [Table 1]
[0127]
[0128] Polymer 1: Actoflow (registered trademark) UT-1001 manufactured by Soken Scientific Co., Ltd., the terminal is modified with a hydroxyl group, and the hydroxyl value is 57±2.
[0129] Polymer 2: Refer to Production Example 1
[01...
manufacture example 1
[0135] A monomer mixture consisting of 65% of 2-ethylhexyl acrylate and 35% of styrene and 0.03 part of 2,2'-azobisisobutyronitrile were dissolved in 700 parts of ethyl acetate in the reactor. After nitrogen substitution, polymerization reaction was performed at 80° C. for 6 hours. The polymerization conversion rate was 95%. The obtained copolymer was dried under reduced pressure, ethyl acetate was evaporated, and a viscous solid copolymer was obtained. The weight average molecular weight of the copolymer was 400,000, and the weight average molecular weight / number average molecular weight was 3.1.
manufacture example 2
[0137] A monomer mixture consisting of 25% n-butyl acrylate and 75% styrene and 0.03 part of 2,2'-azobisisobutyronitrile were dissolved in 700 parts of ethyl acetate in the reactor. After nitrogen substitution, polymerization reaction was performed at 80° C. for 6 hours. The polymerization conversion rate was 95%. The obtained copolymer was dried under reduced pressure, ethyl acetate was evaporated, and a viscous solid copolymer was obtained. The copolymer had a weight average molecular weight of 40,000.
[0138] [Table 2]
[0139]
[0140] [table 3]
[0141]
[0142] Ruthenium catalyst: VC843 manufactured by RIMTEC Co., Ltd.
[0143] The release films of Examples 1 to 9 had high releasability and mechanical strength. The release film of Comparative Example 1 obtained by ring-opening metathesis polymerization of a polymerizable composition containing no polymer containing an alkyl group having 3 or more carbon atoms ( Alkyl meth)acrylate units. Although the alkyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com