Antineoplastic pentacyclic triterpenoid and extraction method thereof
A technology for pentacyclic triterpenes and an extraction method, which is applied in the field of anti-tumor pentacyclic triterpenoids, can solve the problems of inability to prevent ovarian cancer metastasis, large side effects of chemotherapy drugs, adverse reactions, etc. Simple method and good antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
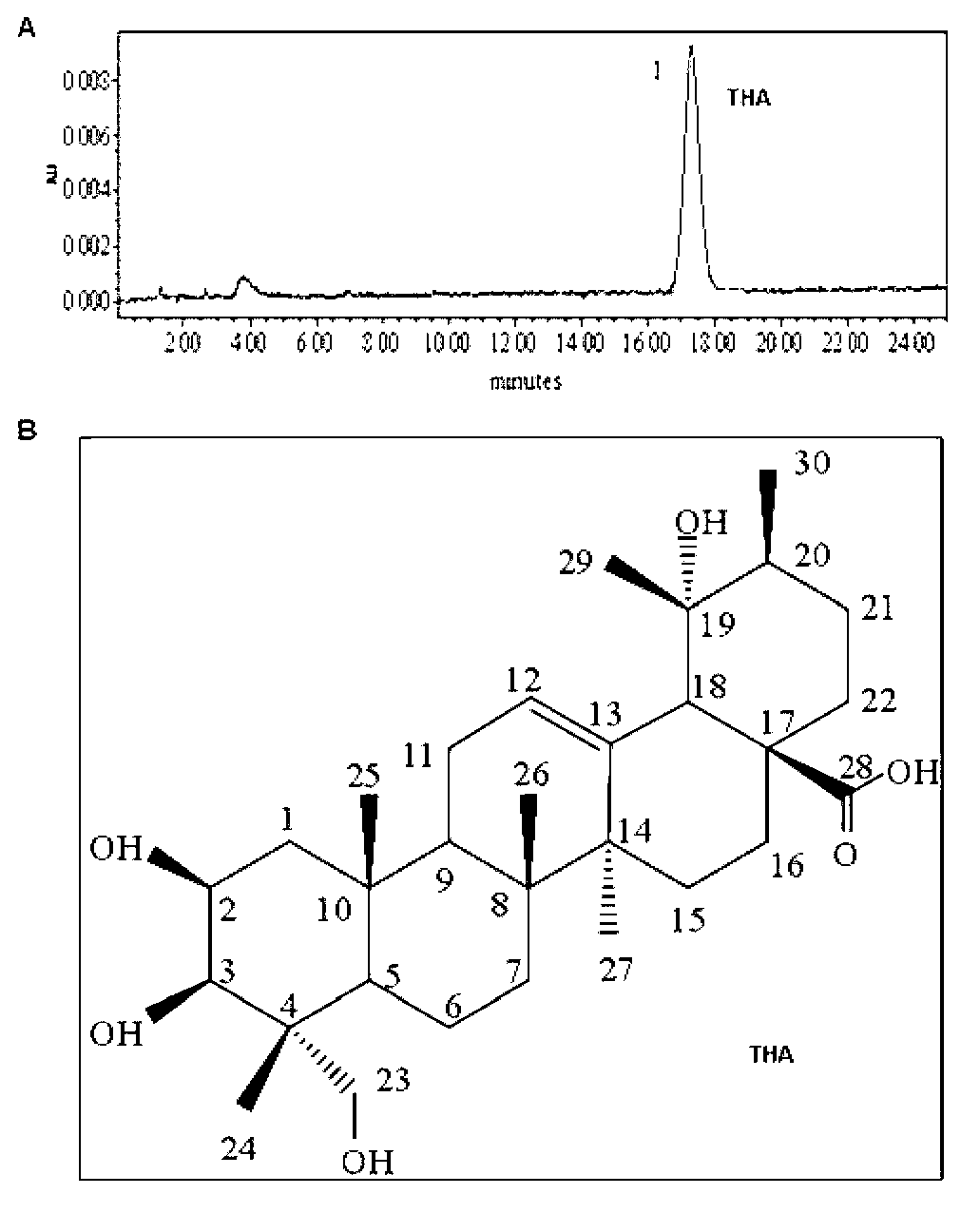
[0028] ① Soak 10 kg of dry leaves of the succulent tree with 20 L of 95% ethanol at room temperature for 3 h, then perform ultrasonic extraction, and the extract is concentrated in a vacuum to obtain a crude extract of about 150 g;
[0029] ② Add the crude extract in ① to distilled water to suspend it in water, and extract with petroleum ether and ethyl acetate to obtain the corresponding extract;
[0030] ③ The 20 g extract obtained in ② was divided into 20 parts through silica gel column chromatography;
[0031] ④ Separate about 4.0 g of the sixth part in ③ using n-hexane-ethyl acetate-ethanol-water by high-speed countercurrent chromatography, and finally obtain about 460 mg of dry powder.
[0032] High-speed countercurrent chromatography is a commonly used technique known to those skilled in the art, and there are existing textbook guidance, such as "High-speed countercurrent chromatography" (author: Zhang Tianyou Wang Xiao , Chemical Industry Press), in addition, the...
experiment example 1
[0035] 1. Experimental method
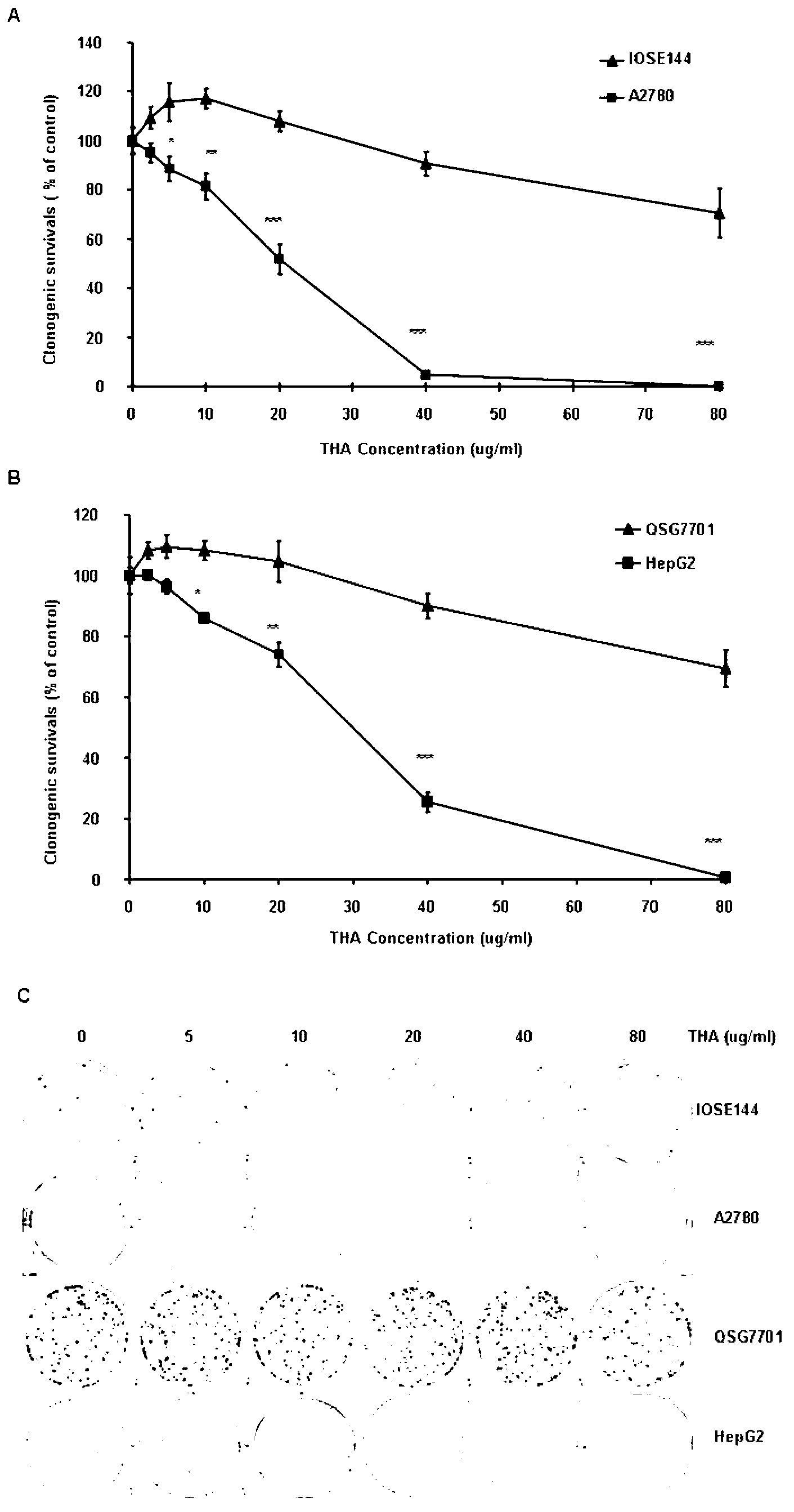
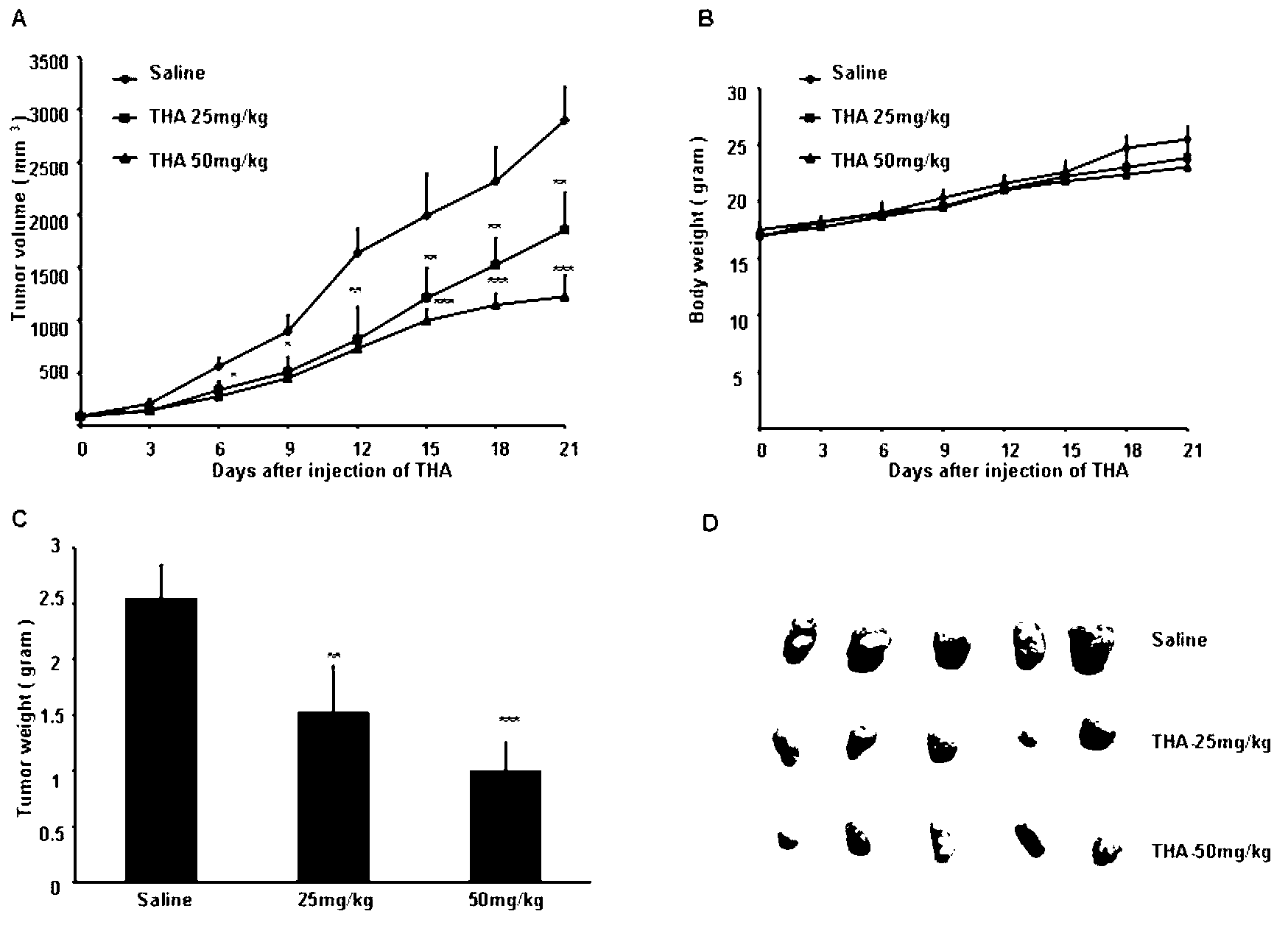
[0036] (1) MTT assay to detect the effect of THA on cell proliferation
[0037] Succinate dehydrogenase in the mitochondria of living cells can reduce exogenous MTT to water-insoluble blue-purple crystal formazan, which is then deposited in the cell, but dead cells have no such function. DMSO can dissolve formazan, and measure its absorbance within a certain range of cell mass. The larger the absorbance value, the stronger the cell activity. In this study, this method was used to detect the effect of THA on cell proliferation. The specific operation is as follows:
[0038] ① Collect logarithmic phase cells and adjust the concentration to 2~5×10 4 cells / 100 μl;
[0039] ② Add 100 μl / well to a 96-well plate, and fill the edge wells with sterile PBS;
[0040] ③a After 24 hours of cell growth, add 2.5, 5, 10, 20, 40 and 80 μg / ml THA to treat the cells, DMSO as a control, and set 3 replicate wells;
[0041] ③b After the cells grew for 24 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com