Copper trifluoromethseleno (I) reagent for aryl halides/alkanes
A halogenated aromatic hydrocarbon and trifluoromethane technology, which is applied in the field of chemical synthesis, can solve the problems of complicated operation, less research on trifluoromethylselenylation method, no single crystal structure, etc., and achieves simple operation and good industrial application prospect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
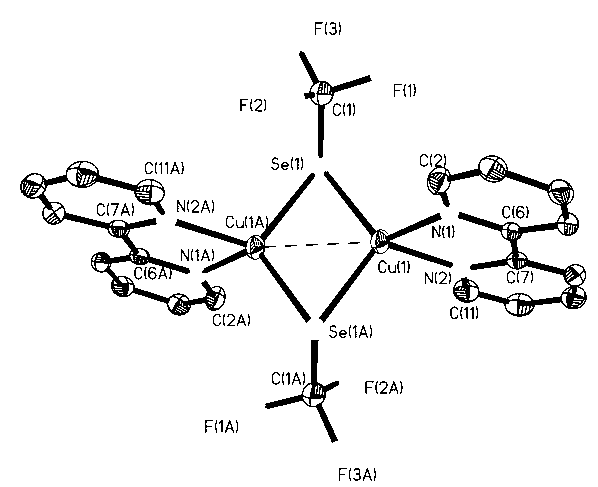
[0021] In a 5ml reactor with a polytetrafluoroethylene cock, add a polytetrafluoroethylene magnetic stirring bar, and weigh 190mg (1.0mmol) CuI, 158mg (2.0mmol) Se powder, 177mg (3.0mmol) anhydrous KF, add 3ml of acetonitrile solvent and mix well, add 450μl (3.0mmol) CF to it with a syringe 3 SiMe 3 , under the protection of nitrogen, stirred overnight at room temperature, filtered with diatomaceous earth, removed the solvent in vacuo, washed three times with anhydrous n-hexane, drained, redissolved with an appropriate amount of acetonitrile, and carefully covered the surface of the solution with 156mg (1.0mmol) 2, The ether solution of 2-bipyridine was placed in a refrigerator at -25°C for 48 hours to obtain 199 mg (53%) of dark red blocky crystals, namely the 2,2-bipyridine trifluoromethylselenyl copper (I) complex. (attached figure 1 It is a single crystal structure, Se(1)-Cu(1) 967(6), Se(1)-C(1) 1.910(3), C(1)-F(1) 1.332(3), C(1 )-F(2) 1.321(4), C(1)-F(3) 1.337(3); 1 ...
Embodiment 2
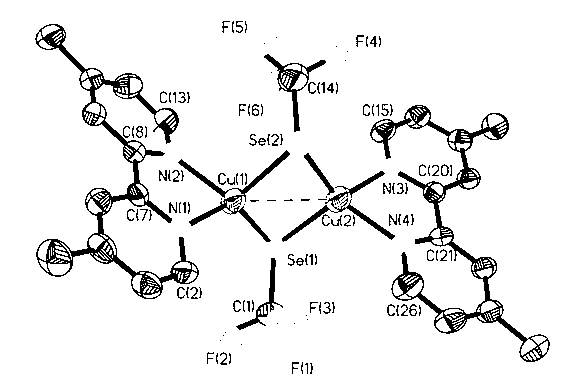
[0023] In a 5ml reactor with a polytetrafluoroethylene cock, add a polytetrafluoroethylene magnetic stirring bar, and weigh 190mg (1.0mmol) CuI, 158mg (2.0mmol) Se powder, 177mg (3.0mmol) anhydrous KF, add 3ml of acetonitrile solvent and mix well, add 450μl (3.0mmol) CF to it with a syringe 3 SiMe 3 , under the protection of nitrogen, stirred overnight at room temperature, filtered with diatomaceous earth, removed the solvent in vacuo, washed three times with anhydrous n-hexane, drained, redissolved with an appropriate amount of acetonitrile, and carefully covered the surface of the solution with 184mg (1.0mmol) 4, The ether solution of 4-dimethyl-2,2-bipyridine was placed in a refrigerator at -25°C for 48 hours to obtain 170 mg (42%) of dark red blocky crystals, namely 4,4-dimethyl-2,2 -Bipyridyl trifluoromethaneselenocopper(I) complexes. (attached figure 2 It is a single crystal structure, Se(1)-Cu(1) 2.3607(11), Se(1)-C(1) 1.904(8), C(1)-F(1) 1.303(10), C(1 )-F(2) 1.29...
Embodiment 3
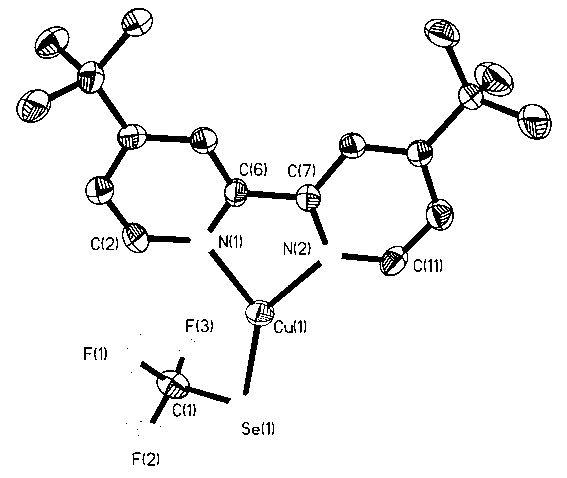
[0025] In a 5ml reactor with a polytetrafluoroethylene cock, add a polytetrafluoroethylene magnetic stirring bar, and weigh 190mg (1.0mmol) CuI, 158mg (2.0mmol) Se powder, 177mg (3.0mmol) anhydrous KF, add 3ml of acetonitrile solvent and mix well, add 450μl (3.0mmol) CF to it with a syringe 3 SiMe 3 , under the protection of nitrogen, stirred overnight at room temperature, filtered with diatomaceous earth, removed the solvent in vacuo, washed three times with anhydrous n-hexane, drained, redissolved with an appropriate amount of acetonitrile, and carefully covered the surface of the solution with 268mg (1.0mmol) 4, The ether solution of 7-di-tert-butyl-2,2-bipyridine was placed in a refrigerator at -25°C for 48 hours, and 220 mg (44%) of orange-red rod-shaped crystals were obtained, namely 4,7-di-tert-butyl-2, 2-Bipyridyl trifluoromethaneselenyl copper (I) complexes. (attached image 3 It is a single crystal structure, Se(1)-Cu(1) 2.2622(14), Se(1)-C(1) 1.913(11), C(1)-F(1)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com