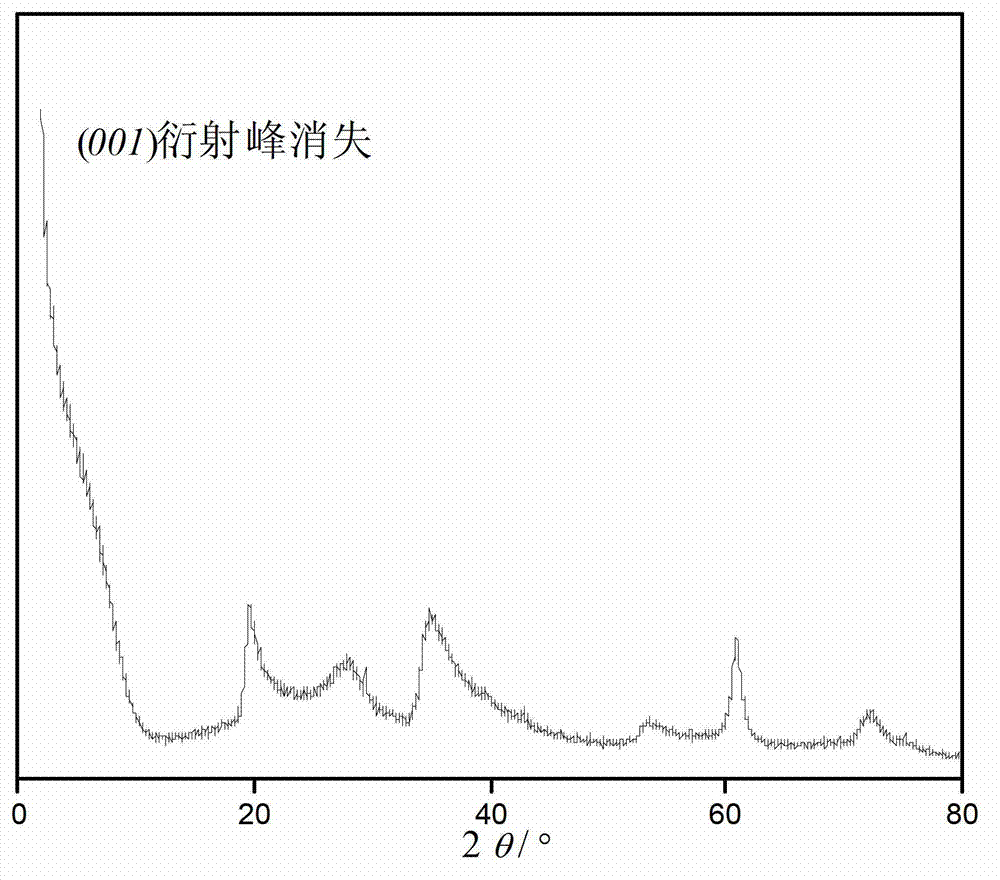
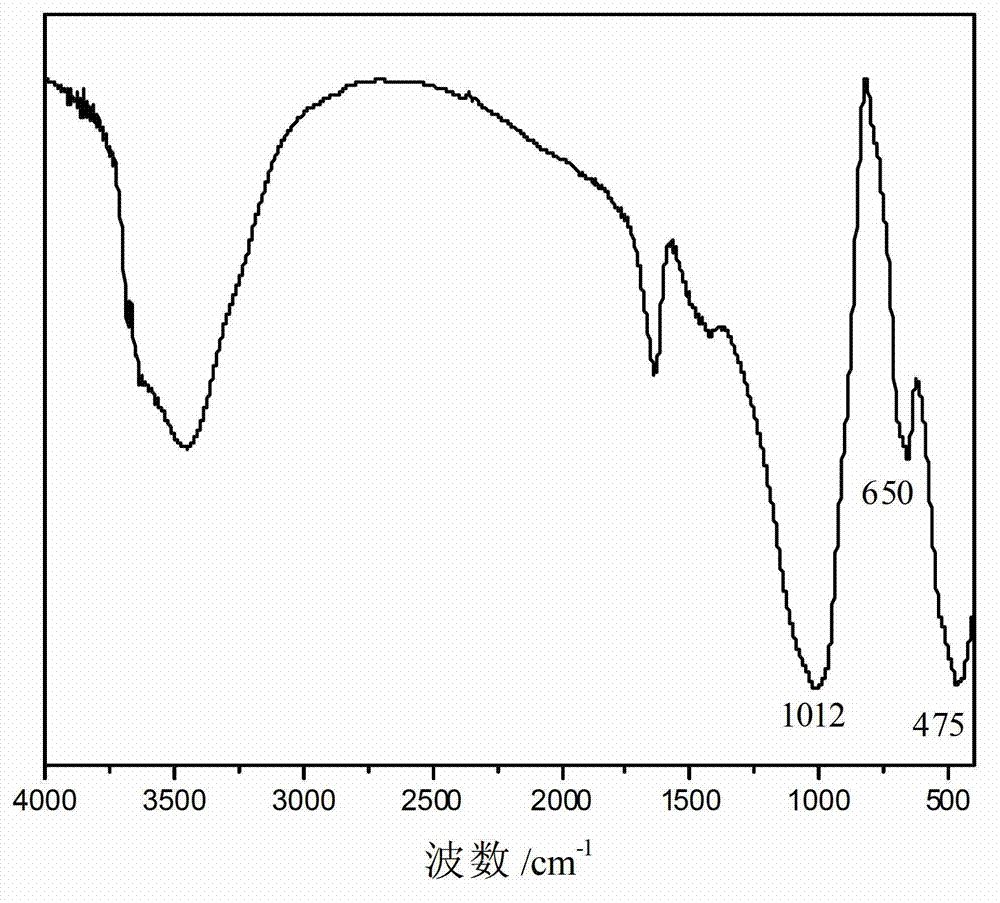
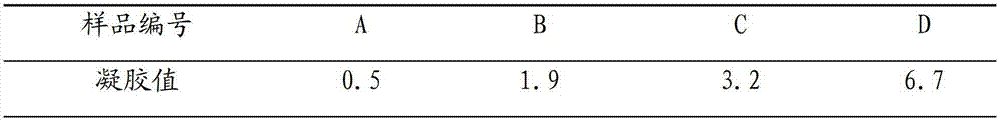
Synthetic method of low-fluorine waterborne rheological additive hectorite
A technology of rheological additives and synthesis methods, applied in the direction of alkali metal silicate, silicate, etc., can solve the problems of human disease hazards, F poisoning, hectorite non-existence hazards, etc., and achieve low fluorine content, Broad economic prospects, excellent thickening effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Take water glass (mass concentration is 28wt%, modulus is 2.2), Mg(NO 3 ) 2 , NaF, NaOH and LiCl, with Si 4+ The number of moles is the standard, let Mg 2+ moles / Si 4+ The value of the number of moles of Li is a, Li + moles / Si 4+ The value of the number of moles of b, OH - moles / Si 4+ The value of the number of moles of c, F - moles / Si 4+ The value of the number of moles is d, so that Mg 2+ , Li + , OH - and F - The number of moles of satisfies the following ratio:
[0041] a=4; b=0.7; c=2; d=0.02
[0042] Prepare the mixed solution according to the above ratio, specifically: (1) Dissolve the soluble magnesium salt and lithium salt with an appropriate amount of water first, and then add the fluorine salt; (2) Dissolve the water glass with an appropriate amount of water to obtain a silicon source solution; use the remaining water Dissolve NaOH to obtain lye for later use; (3) Slowly drop the silicon source solution and lye into the boiling solution in step ...
Embodiment 2
[0049] Take water glass (mass concentration is 28wt%, modulus is 2.2), Mg(NO 3 ) 2 、Na 2 CO 3 and LiCl, with Si 4+ The number of moles is the standard, let Mg 2+ moles / Si 4+ The value of the number of moles of Li is a, Li + moles / Si 4+ The value of the number of moles of b, OH - moles / Si 4+ The value of the number of moles of c, F - moles / Si 4+ The value of the number of moles is d, so that Mg 2+ , Li + , OH - and F - The number of moles of satisfies the following ratio:
[0050] a=3; b=0.8; c=2; d=0
[0051] Prepare the mixed solution according to the above ratio, specifically: (1) Dissolve the soluble magnesium salt and lithium salt with an appropriate amount of water first, and then add the fluorine salt; (2) Dissolve the water glass with an appropriate amount of water to obtain a silicon source solution; use the remaining water dissolved Na 2 CO 3 Obtain lye for later use; (3) Slowly drop the silicon source solution and lye into the boiling solution in st...
Embodiment 3
[0056] Take Na + Stabilized Silica Hydrosol, MgCl 2 、Na 2 CO 3 and LiCl, with Si 4+ The number of moles is the standard, let Mg 2+ moles / Si 4+ The value of the number of moles of Li is a, Li + moles / Si 4+ The value of the number of moles of b, OH - moles / Si 4+ The value of the number of moles of c, F - moles / Si 4+ The value of the number of moles is d, so that Mg 2+ , Li + , OH - and F - The number of moles of satisfies the following ratio:
[0057] a=4; b=1; c=1; d=0
[0058] Prepare the mixed solution according to the above ratio, specifically: (1) Dissolve soluble magnesium salt and lithium salt with appropriate amount of water first, and then add fluorine salt; (2) Dissolve Na with appropriate amount of water + Stable silica hydrosol to obtain silicon source solution; use remaining water to dissolve Na 2 CO 3 Obtain lye for later use; (3) Slowly drop the silicon source solution and lye into the boiling solution in step (1), make the system condense and re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com