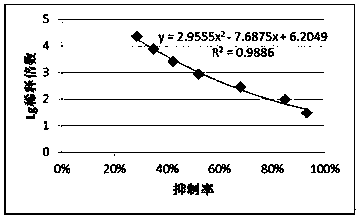
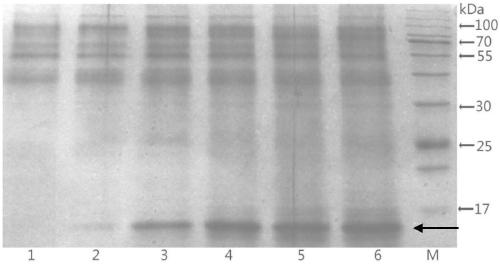
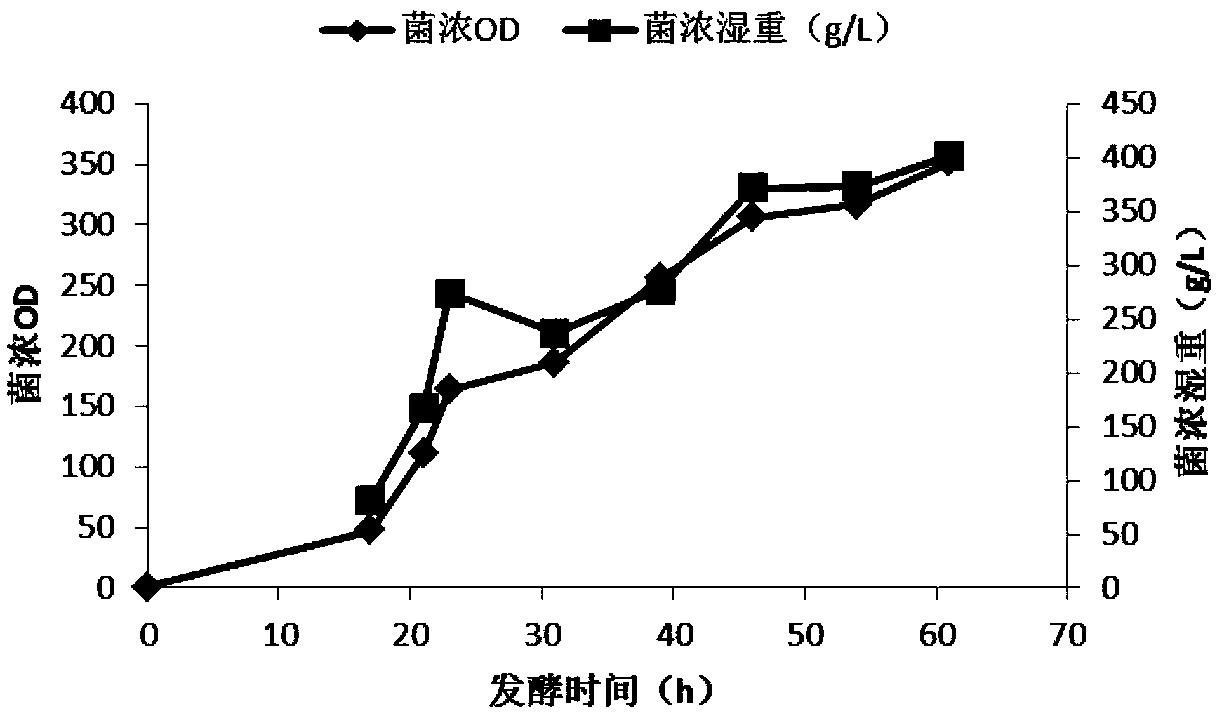
Preparation method and application of restructured dust mite II type allergen Der p2 and Der f2 protein
An allergen and dust mite technology, which is applied in the field of preparation of recombinant type II allergen Derp2 and Derf2 proteins, can solve problems such as incorrect structure of DF2 protein, difficult to meet clinical dosage, weak serum reactivity, etc. Achieve the effects of increased yield, improved activity, and simple ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1 recombinant DP2 / DF2 small-scale (3L) high-density fermentation process
[0057] Step 1: Activation of recombinant strains
[0058] frozen at -80°C DP2 Glycerol bacteria seeds in the working seed bank were streaked on YPD solid medium (yeast extract 10g / L, peptone 20g / L, glucose 20g / L, agarose 15g / L), and cultured in a constant temperature and humidity box at 30°C for 3-5 days .
[0059] Step 2: Seed liquid culture
[0060] Pick the monoclonal colonies on the solid medium and culture them in YPD liquid medium (yeast extract 10g / L, peptone 20g / L, glucose 20g / L) at 30°C and 220rpm until OD 600 ≈6.0, and observe under the microscope that there is no miscellaneous bacteria, and the seed liquid for fermentation is obtained.
[0061] Step 3: Fermentation Process
[0062] Clean the Sartorius B-plus bioreactor, calibrate the pH meter probes of the fermenter with pH=7.0 and pH=4.0 standard solutions, and then configure BSM medium as the fermentation med...
Embodiment 2
[0068] Example 2 Recombinant DP2 / DF2 large-scale (30L) high-density fermentation verification
[0069] Step 1: Activation of recombinant strains
[0070] Streak the Glycerol bacteria seeds in the working seed bank frozen at -80°C on YPD solid medium (yeast extract 10g / L, peptone 20g / L, glucose 20g / L, agarose 15g / L) and keep the temperature at 30°C Cultivate in a humid chamber for 3-5 days.
[0071] Step 2: Primary seed liquid culture
[0072] Pick the monoclonal colony on the solid medium in step 1 and put it in YPD liquid medium (yeast extract 10g / L, peptone 20g / L, glucose 20g / L), 30°C, 220rpm and cultivate to OD 600 ≈6.0, and observed under a microscope without any bacteria, the first-grade seed liquid for fermentation was obtained.
[0073] Step 3: Secondary Seed Solution Culture
[0074] Inoculate the primary seed liquid obtained in step 2 into the Sartorius B-plus fermenter, and cultivate it with 60% BSM, pH=5.5±0.2, 27°C, 300rpm, and maintain dissolved oxygen...
Embodiment 3
[0079] Example 3 Recombinant DP2 / DF2 Pilot Test (300L) High Density Fermentation Verification
[0080] Step 1: Activation of recombinant strains
[0081] Streak the Glycerol bacteria seeds in the working seed bank frozen at -80°C on YPD solid medium (yeast extract 10g / L, peptone 20g / L, glucose 20g / L, agarose 15g / L) and keep the temperature at 30°C Cultivate in a humid chamber for 3-5 days.
[0082] Step 2: Primary seed liquid culture
[0083] Pick the monoclonal colony on the solid medium in step 1 and put it in YPD liquid medium (yeast extract 10g / L, peptone 20g / L, glucose 20g / L), 30°C, 220rpm and cultivate to OD 600 ≈6.0, and observed under a microscope without any bacteria, the first-grade seed liquid for fermentation was obtained.
[0084] Step 3: Secondary Seed Solution Culture
[0085] Inoculate the primary seed solution obtained in step 2 into a Diwoxin BVT-3000 30L seed tank, and cultivate it with 60% BSM, pH=5.5±0.2, 27°C, and 300rpm, and dissolve it throu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com