Method for preparation of barium sulfate porous material with different microstructures by taking barium peroxide as barium source
A technology of barium peroxide and porous materials, applied in the direction of calcium/strontium/barium sulfate, etc., can solve the problems of limited barium sulfate, backward production technology, low variety structure and grade, and achieve controllable structure, no three waste discharge, Energy saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
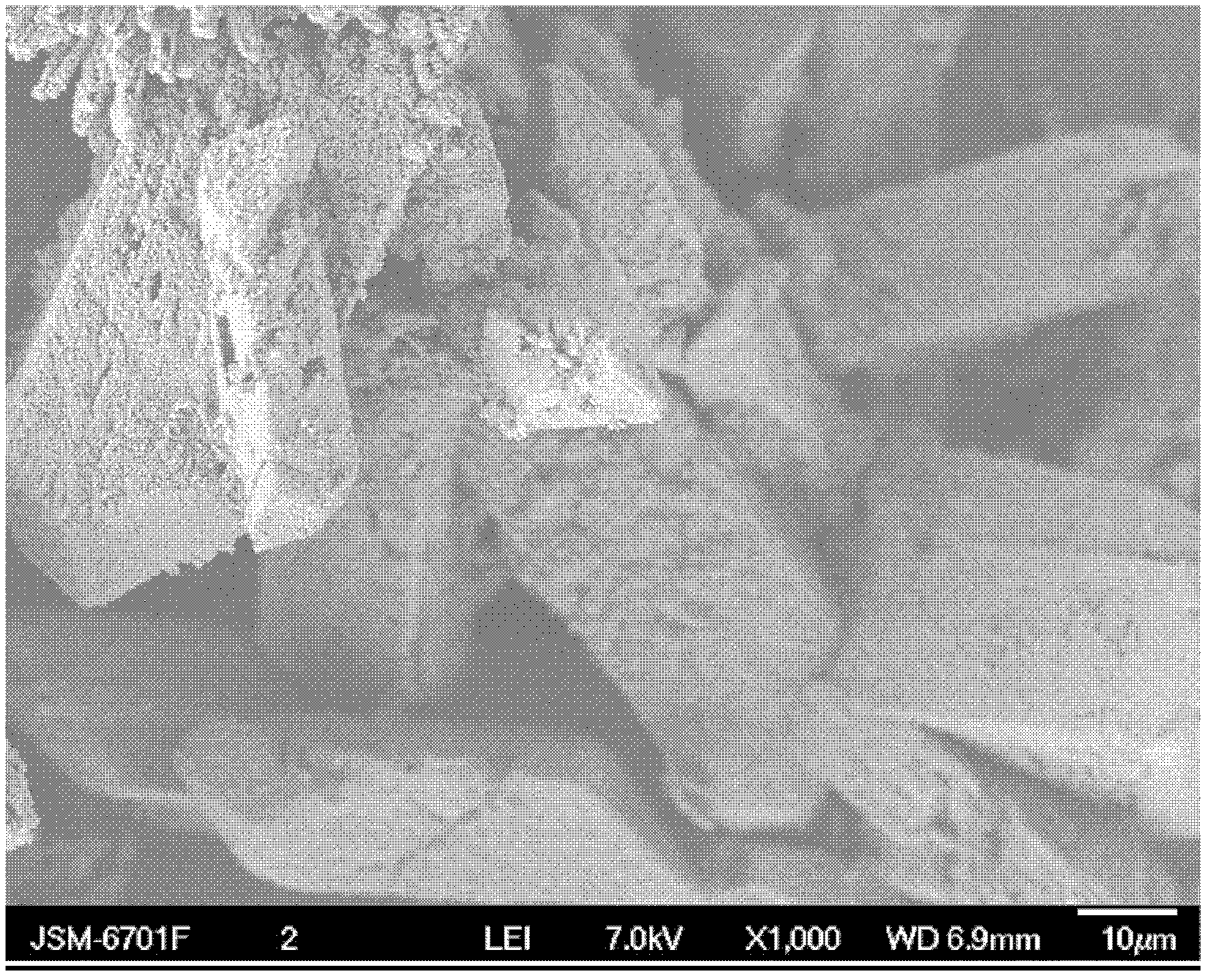
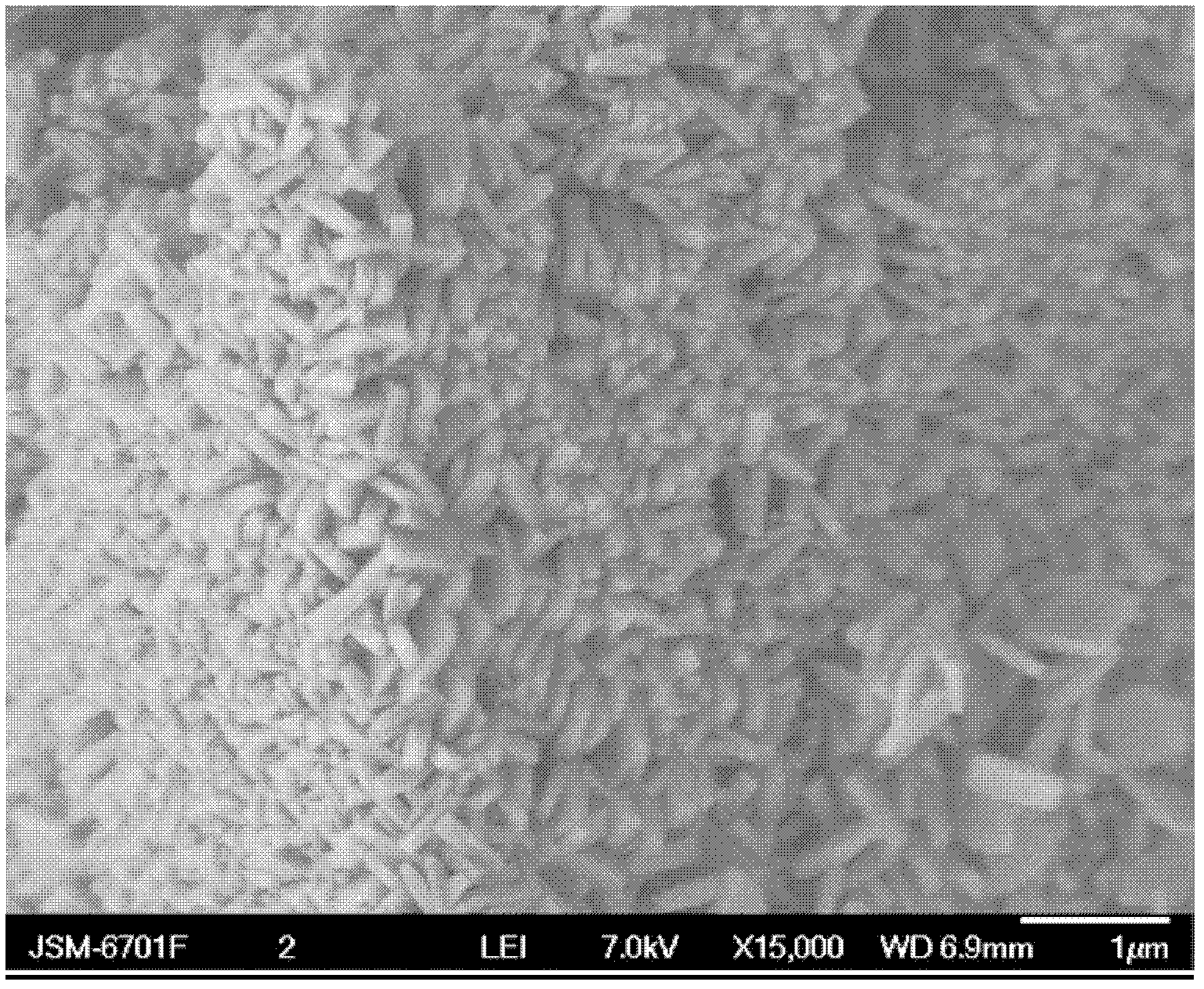
[0028] After cleaning the 1L glass beaker, add sodium hydroxide aqueous solution to 0.3% hydrogen peroxide aqueous solution in 1000ml mass concentration, adjust the pH value of hydrogen peroxide aqueous solution to 11, add 435g ammonium sulfate to the sulfate radical in the solution under stirring The concentration of the solution is 3.3 mol / L; after the ammonium sulfate is completely dissolved, 100 g of barium peroxide is added, and aged at room temperature for 5 hours; filtered and dried to obtain a barium sulfate porous material with a hexagonal microstructure. The pore diameter of the obtained barium sulfate porous material is about 10 nm to 100 nm.
[0029] The SEM photo and microstructure morphology of the prepared barium sulfate porous material are shown in Figure 1a and Figure 1b .
Embodiment 2
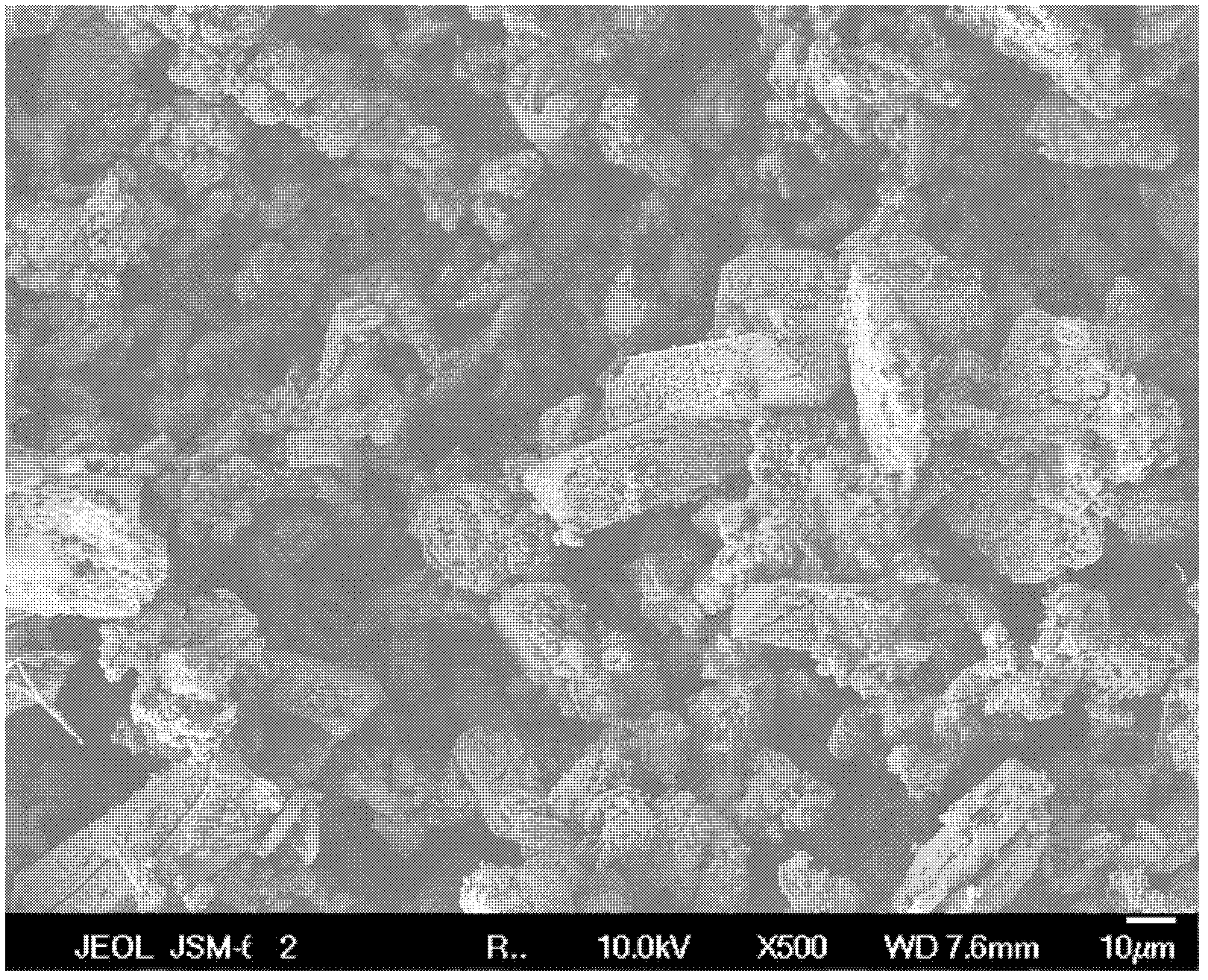
[0031] After cleaning the 1L glass beaker, add sodium polystyrene sulfonate in 5% hydrogen peroxide aqueous solution in 1000ml mass concentration, make the concentration of polystyrene sodium sulfonate in the hydrogen peroxide aqueous solution be 0.2g / L, with Adjust the pH value of the solution with ammonia water to 13.5, add 1.74g of potassium sulfate under stirring until the concentration of sulfate radicals in the solution is 0.01mol / L; put 1g of barium peroxide into the solution after the potassium sulfate is completely dissolved, and age at room temperature for 72 hours; centrifuge and dry , to obtain barium sulfate porous material with spherical microstructure. The pore diameter of the obtained barium sulfate porous material is about 100 nm to 600 nm.
[0032] The SEM photo and microstructure morphology of the prepared barium sulfate porous material are shown in Figure 2a and Figure 2b .
Embodiment 3
[0034] After cleaning the 1L glass beaker, add sodium polyacrylate in 1000ml of aqueous hydrogen peroxide solution with a mass concentration of 0.9%, so that the concentration of sodium polyacrylate in aqueous hydrogen peroxide solution is 1g / L, adjust the solution with aqueous potassium hydroxide solution The pH value of the solution is 12, under stirring, add 14.2g of magnesium sulfate to the concentration of the sulfate group in the solution to be 0.1mol / L; after the magnesium sulfate is completely dissolved, drop into 10g of barium peroxide, leave it at room temperature for aging for 48 hours; suction and dry to obtain Barium sulfate porous material with rod-like microstructure. The pore diameter of the obtained barium sulfate porous material is about 30nm-100nm.
[0035] The SEM photo and microstructure morphology of the prepared barium sulfate porous material are shown in Figure 3a and Figure 3b .
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com