Preparation method of CuInSe2 with a chalcopyrite structure and CuIn1-xGazSe2 nano particles
A cuin1-xgaxse2 and nanoparticle technology is applied in the field of preparation of CuInSe2 and CuIn1-xGaxSe2 nanoparticles with chalcopyrite structure, which can solve problems such as difficulty in mass production, and achieve simple operation, strong repeatability and short synthesis cycle. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The implementation process of Embodiment 1 of the present invention is as follows:
[0028] (1). Weigh 170mg CuCl 2 2H 2 O (1 mmol) and 293 mg InCl 3 4H 2 O (1 mmol) was placed in a 50 ml three-necked flask, 15 ml of oleylamine (OLA) was added, heated to 60 °C and magnetically stirred for 3 h, after the solution was clarified, it was naturally cooled to room temperature to obtain a transparent yellow metal precursor solution.
[0029] (2). Weigh 158 mg of elemental Se powder (2 mmol) into another 50 ml three-necked flask, add 10 ml of oleylamine (OLA), stir magnetically at room temperature for 0.5 h, and oscillate ultrasonically for 10 min to obtain a Se powder precursor solution.
[0030] (3). Mix the metal precursor solution and the Se powder precursor solution, and place the resulting mixed solution in a three-neck flask connected to the Schlenk line.
[0031] (4). At room temperature, vacuumize the three-necked flask for 5 minutes, then heat the resulting mixed ...
Embodiment 2
[0039] The reaction temperature in the step (4) of embodiment two is 230 o C, the reaction time is 1h, and other steps are the same as the corresponding steps of Example 1.
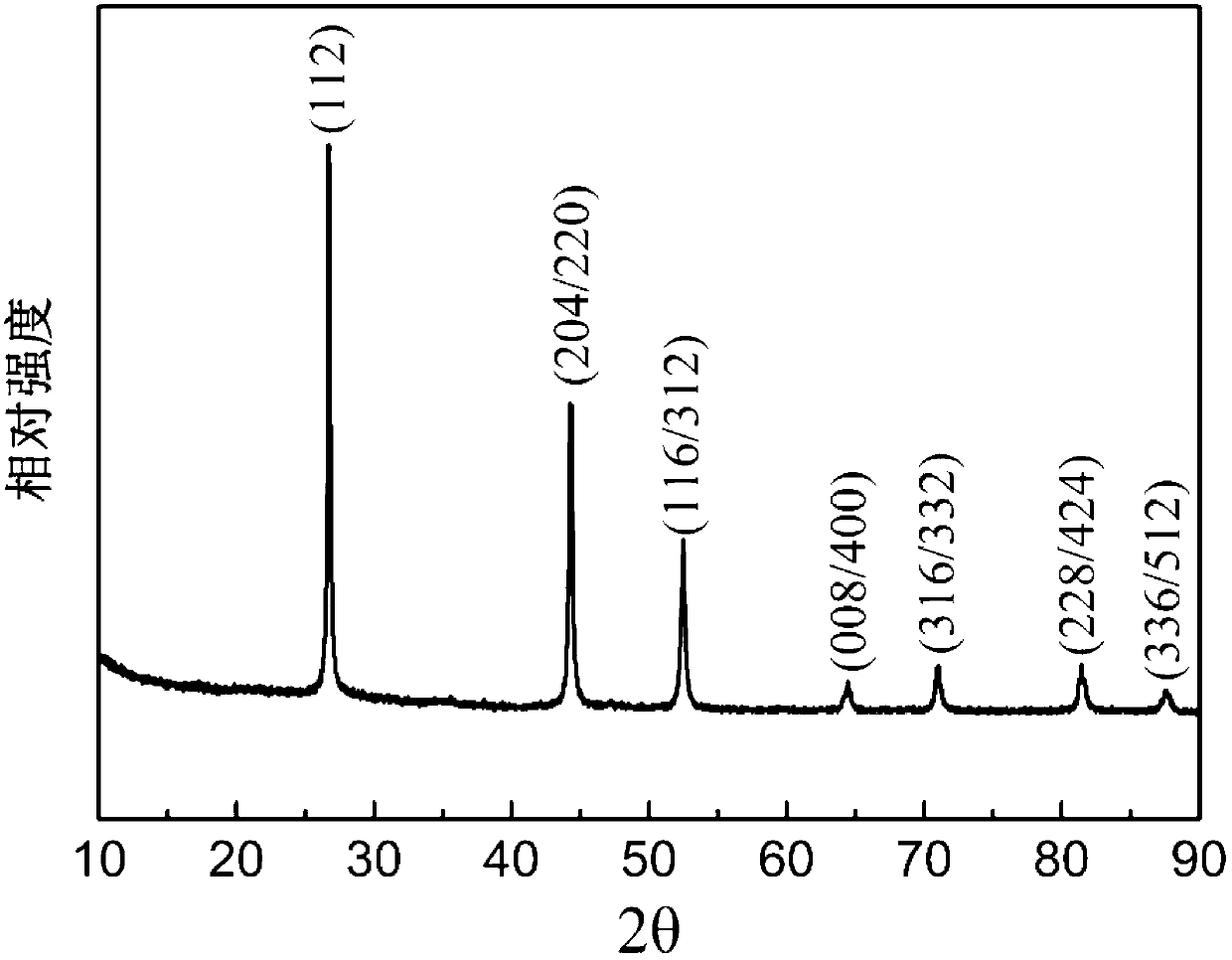
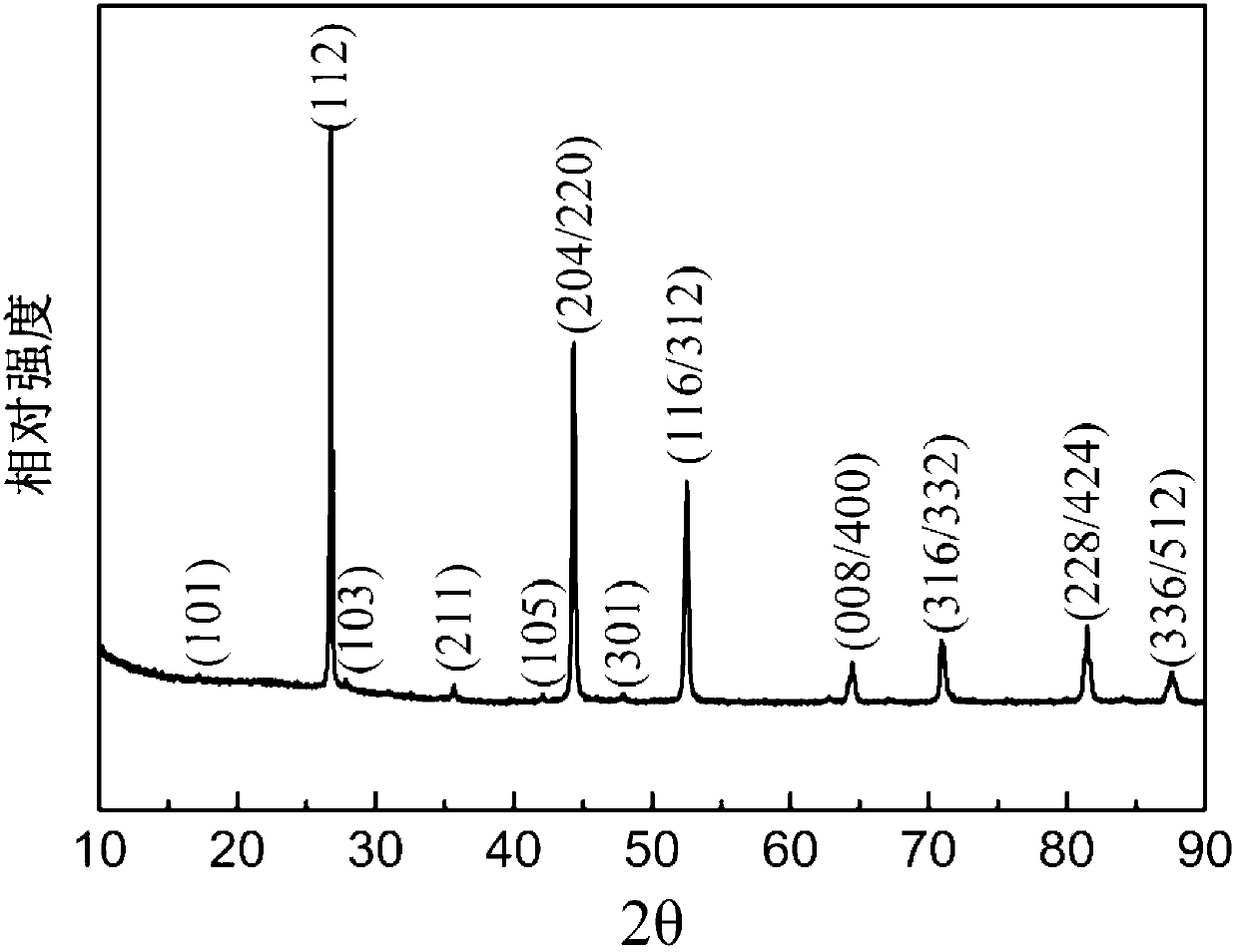
[0040] figure 2 For the CuInSe prepared by the method of embodiment two 2 XRD patterns of nanoparticles.
[0041] Such as figure 2 Shown, CuInSe 2 All diffraction peaks of nanoparticles are related to chalcopyrite CuInSe 2 The standard card JCPDF No.40-1487 corresponds very well, indicating that the CuInSe made by the preparation method of Example 2 2 The nanoparticles have a chalcopyrite crystal structure and are very crystalline.
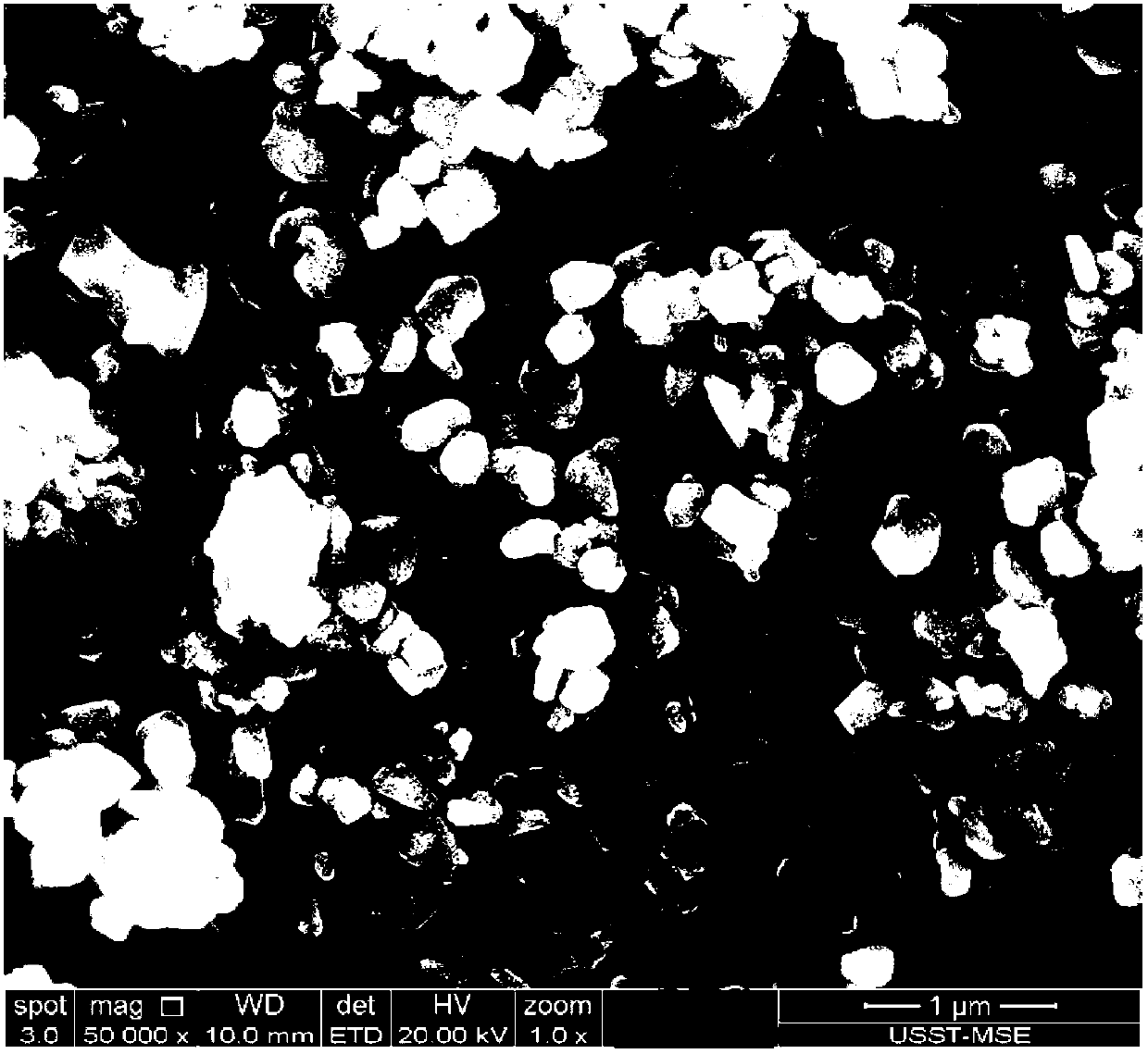
[0042] image 3 For the CuInSe prepared by the method of embodiment two 2 SEM pattern of nanoparticles.
[0043] Such as image 3 Shown, CuInSe 2 The dispersion of nanoparticles is very good, and the particle size is between 50-300 nm.
Embodiment 3
[0045] The implementation process of embodiment three of the present invention is as follows:
[0046] (1). Weigh 119.3mg CuCl 2 2H 2 O (0.7 mmol) and 205.3 mg InCl 3 4H 2 O (0.7mmol) and put in a 50ml three-necked flask, add 15ml oleylamine (OLA), heat to 50 O C and magnetically stirred for 4 h, after the solution was clear, it was naturally cooled to room temperature to obtain a transparent yellow metal precursor solution.
[0047] (2). Weigh 110.5mg of elemental Se powder (1.4mmol) into another 50ml three-necked flask, add 10ml of oleylamine, stir magnetically at room temperature for 1h, and oscillate ultrasonically for 10min to obtain a Se powder precursor solution.
[0048] (3). Mix the metal precursor solution and the Se powder precursor solution, and place the resulting mixed solution in a three-neck flask connected to the Schlenk line.
[0049] (4). At room temperature, vacuumize the three-necked flask for 5 minutes, then heat the resulting mixed solution with an ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com