Method for detecting H1N1 influenza A virus variant by multiple target point gene and kit
A technology for influenza virus and gene detection, applied in the field of primers for multi-target detection, which can solve the problems of false negatives or false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Preparation of a multi-gene target detection kit for influenza A (H1N1) 2009 mutant strains.
[0073] 1) Synthesize an oligodeoxynucleic acid primer, the sequence of which is shown in SEQ ID NO: 1-8 above, and the 5' end of the antisense primer is labeled with a reporter fluorescent group FAM (6-carboxy-fluorescein).
[0074] 2) Purchase RT-PCR enzyme and DNA polymerase.
[0075] 3) Configure the reaction solution.
[0076] 4) Configure the positive control and negative control and dilute to the optimum concentration.
[0077] Table 4 kit preparation composition
[0078] Composition (50tests / box)
quantity
RT-PCR reaction solution
1.5ml
RT-PCR enzyme 100U / ul
10ul
PCR reaction solution
1.5ml
Taq DNA polymerase 5U / ul
15ul
Molecular Biological Purity Water
2ml
1ml
1ml
[0079] The specific material composition is as described above...
Embodiment 2
[0085] Example 2 Application of Multi-gene Target Detection Kit for Influenza A H1N1 Influenza Virus 2009 Variant Strain
[0086] 1) Sample processing (RNA extraction)
[0087] Use Qiagen's QIAamp Viral RNA Mini Kit, or other corresponding kits, to extract from samples collected from humans. Performed in the sample handling area. Samples were stored at -80°C.
[0088] 2) Amplification reagent preparation and reaction process
[0089] RT-PCR: Take out the RT-PCR reaction solution and RT-PCR enzyme from the kit, melt at room temperature, and centrifuge at 2,000g for 5sec. Assuming that the number of PCR tubes required is n (n=number of samples+1 tube of negative control+1 tube of positive control), each RT-PCR test reaction system is 20 μL. Calculate the amount of each reagent used, add it to a test tube of appropriate volume, mix well, dispense 15 μL into each PCR tube, and transfer to the sample processing area. Add 5 μL of each prepared RNA solution to each set PCR tube,...
Embodiment 3
[0096] Embodiment 3, the specificity test of kit
[0097] 1) Materials, as listed in Table 5, 322 known clinical samples.
[0098] 2) method
[0099] Clinical samples were tested with the diagnostic kit prepared in Example 1 to verify the specificity of the method.
[0100] 3) Results
[0101] As shown in Table 5, the results indicated that the established method had high specificity and produced no false positive or false negative results.
[0102] The test results are shown in Table 5.
[0103] Table 5 Test results of clinical samples
[0104]
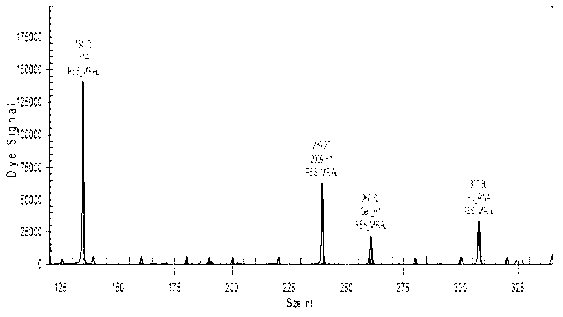
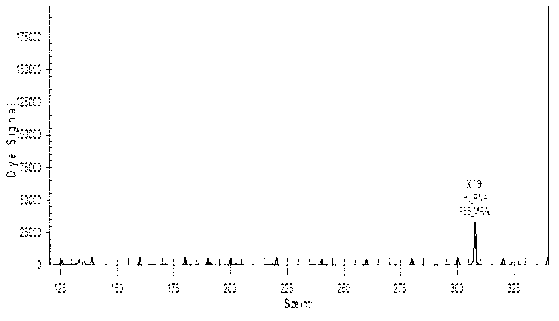
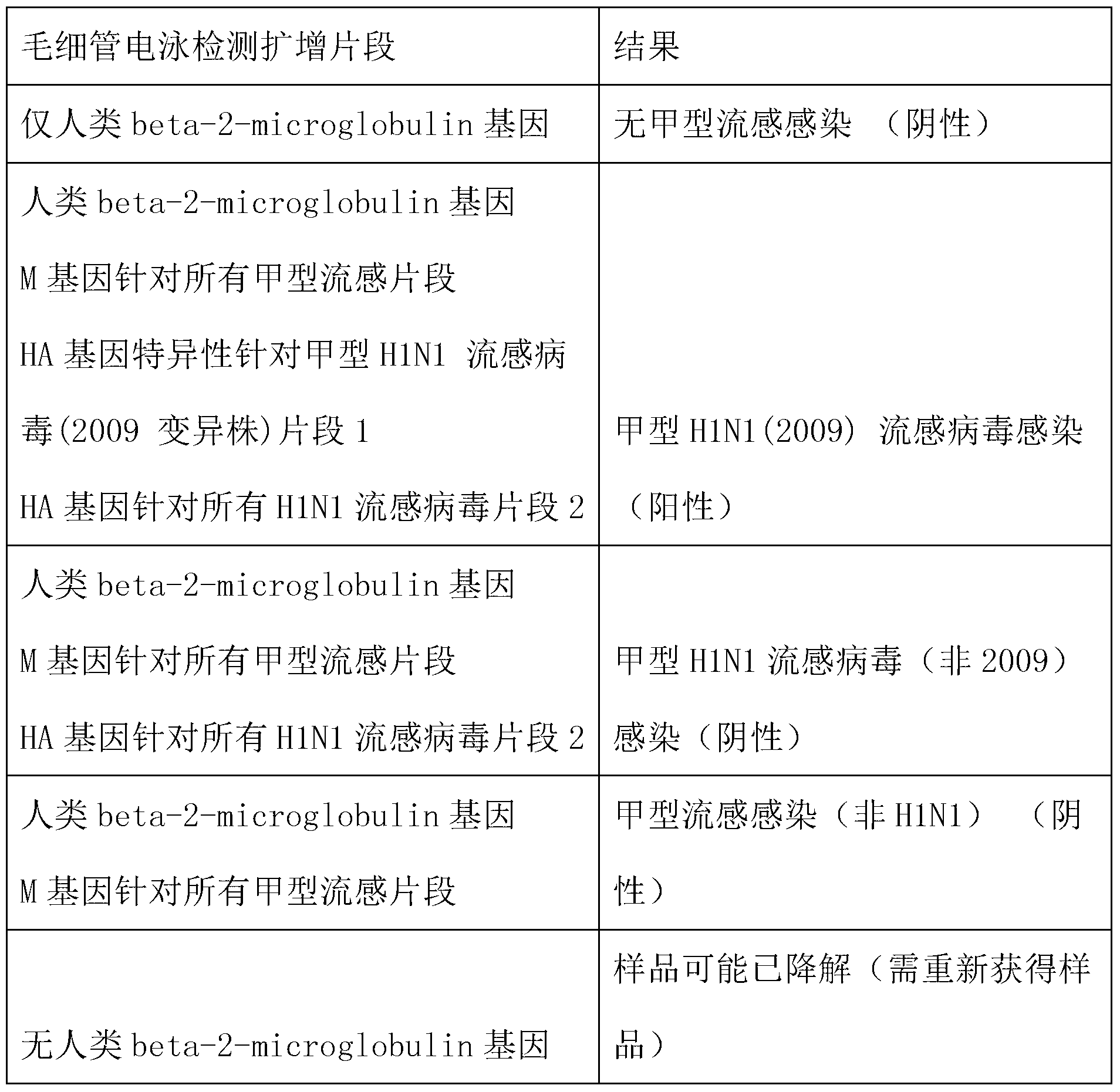
[0105] In Table 5, detection gene segment A is the human beta-2-microglobulin gene, segment B is the M gene for all influenza A, segment C is the HA gene for all H1N1 influenza, and segment D is the HA gene for influenza A 2009H1N1.
[0106] As can be seen from the results in Table 5, 322 known samples are detected, and the results show that the established method can detect all of the influenza A H1N1 influenza virus (2009 v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com