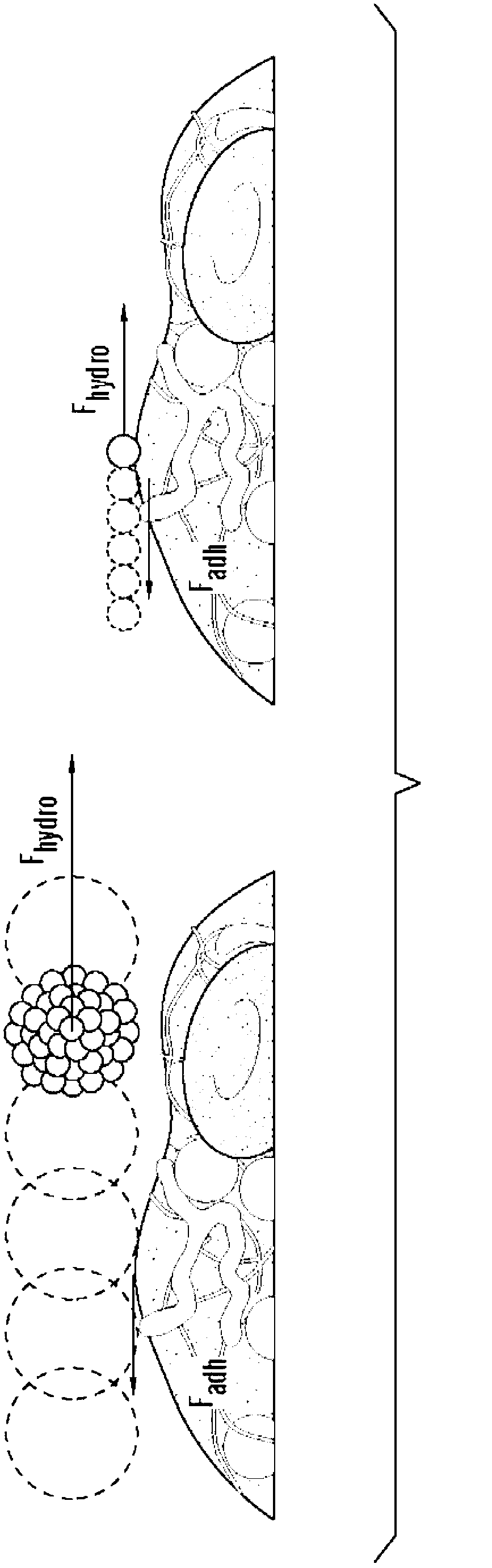
Shear controlled release for stenotic lesions and thrombolytic therapies
A thrombus and anti-thrombotic technology, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, sexual diseases, etc., and can solve the problems that have not been proposed or developed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0452] PEG-PLGA (50:50MW4:17kDA) or PLGA (50:50MW17kDa) nanoparticles were prepared as follows. Using maleimide-PEG-NH 2 Linkers, COOH-PEG-PLGA or COOH-PLGA polymers were conjugated to binding peptides (CREKA (SEQ ID NO: 1) or CRKRLDRNK (SEQ ID NO: 3)). First, the cysteine terminus of the peptide was conjugated to the maleimide terminus of the PEG linker in PBS buffer at pH 6.5. Second, the carboxyl-functionalized PEG-PLGA polymer was preactivated with 1-ethyl-3-(3-dimethylaminopropyl) (EDC) and N-hydroxysuccinimide (NHS), followed by the corresponding The PEG-modified peptides were reacted in PBS / DMSO mixture for about 4 hours at room temperature. The final product was purified by dialysis and a lyophilized powder was obtained. conjugation reaction by 1 Confirmed by H NMR. The peptide-conjugated polymer was then dissolved with coumarin (a hydrophobic dye) in ethyl acetate or DMSO, and the nanoparticles were prepared by w / o / w emulsion method or simple solvent displaceme...
Embodiment 2
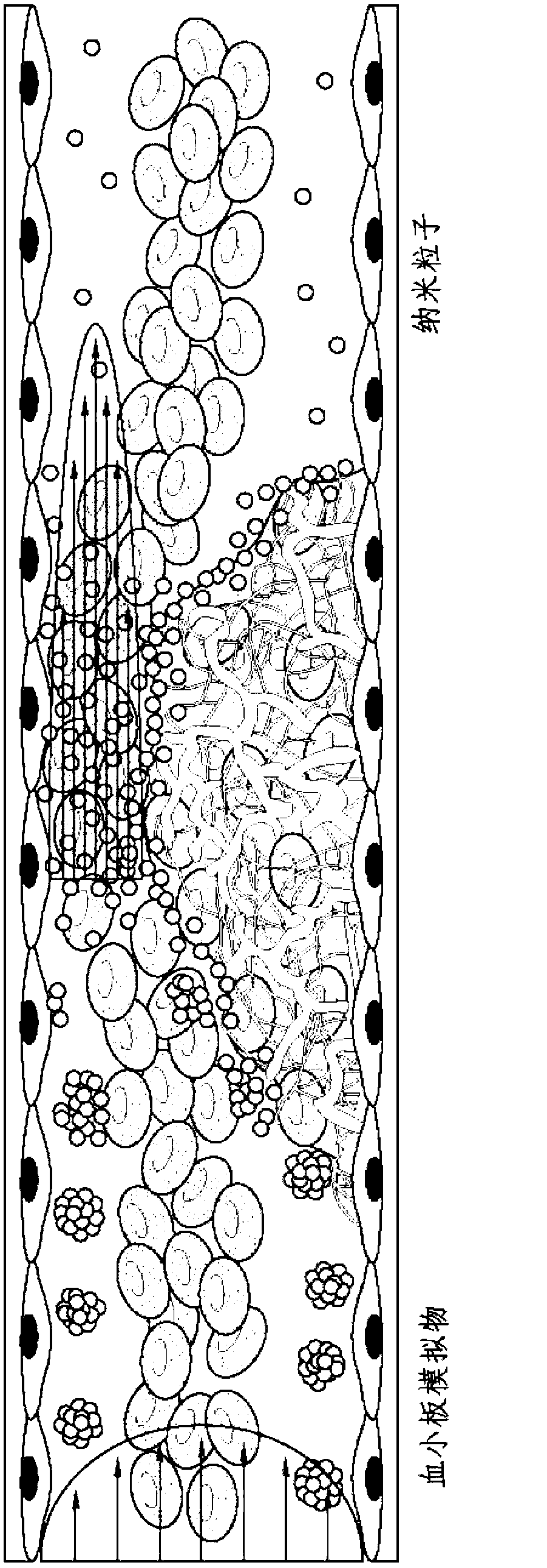
[0457] Example 2: Shear-Activated Platelet Mimetics for Targeting Drugs to Obstruct Blood Vessels
[0458] Materials and methods
[0459] Nanoparticle preparation: Nanoparticles (NP) were prepared from PLGA (50:50, 17 kDa, acid-terminated; Lakeshore Biomaterials, AL) using a simple solvent exchange method (C.E. Astete & C.M. Sabliov, Journal of Biomaterials Science, Polymer Edition 17, 247( 2006)). The fluorescent hydrophobic dye coumarin was included in the NPs to enable visualization and quantification in this study. Briefly, 1 mg / ml of polymer was dissolved with coumarin in dimethylsulfoxide (DMSO, Sigma, MO), dialyzed against water at room temperature, and the nanoparticles were formed by solvent displacement. The size distribution and morphology of the formed NPs were characterized using dynamic light scattering (DLS), scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
[0460] Manufacture of platelet mimics - PLGA NPs were centrifuged and...
Embodiment 3
[0494] Example 3: Shear Stress Controlled Release from RBCs
[0495] Red blood cell ghost cells were prepared using hypotonic hemolysis. Briefly, RBCs were centrifuged from blood (2000 g, 10 min) and resuspended in calcium / magnesium-free diluted PBS (1:10 volume ratio of PBS to DD water). The cells were incubated at 4°C for 15 minutes and then centrifuged (12,000 g, 10 min). This process was repeated four times. The cells were subsequently loaded with FITC-dextran by incubating the cells with 5 mg / ml of dextran in diluted PBS for 1 hour at 4°C. The cells were centrifuged, suspended in PBS buffer containing calcium / magnesium, and allowed to reseal over two hours in a 37°C incubator. After the reclosure procedure, the cells were washed four times in PBS to remove any residues in solution. Figure 8 Fluorescence images of FITC-dextran-loaded RBC ghost cells are shown, imaged 5 days after preparation of the FITC-dextran-loaded RBC ghost cells.
[0496] Inject a suspension of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com