Method for synthesizing 3-methyl-3-butenyl-1-ol by two-step process
A technology of methyl and butene, applied in chemical instruments and methods, preparation of organic compounds, preparation of oxygen-containing compounds, etc., can solve problems such as high reaction temperature and pressure, large amount of catalyst and organic solvent, excessive isobutene, etc. Achieve the effect of reducing energy consumption and material consumption of separation, reducing the difficulty of separation and processing, and reducing loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
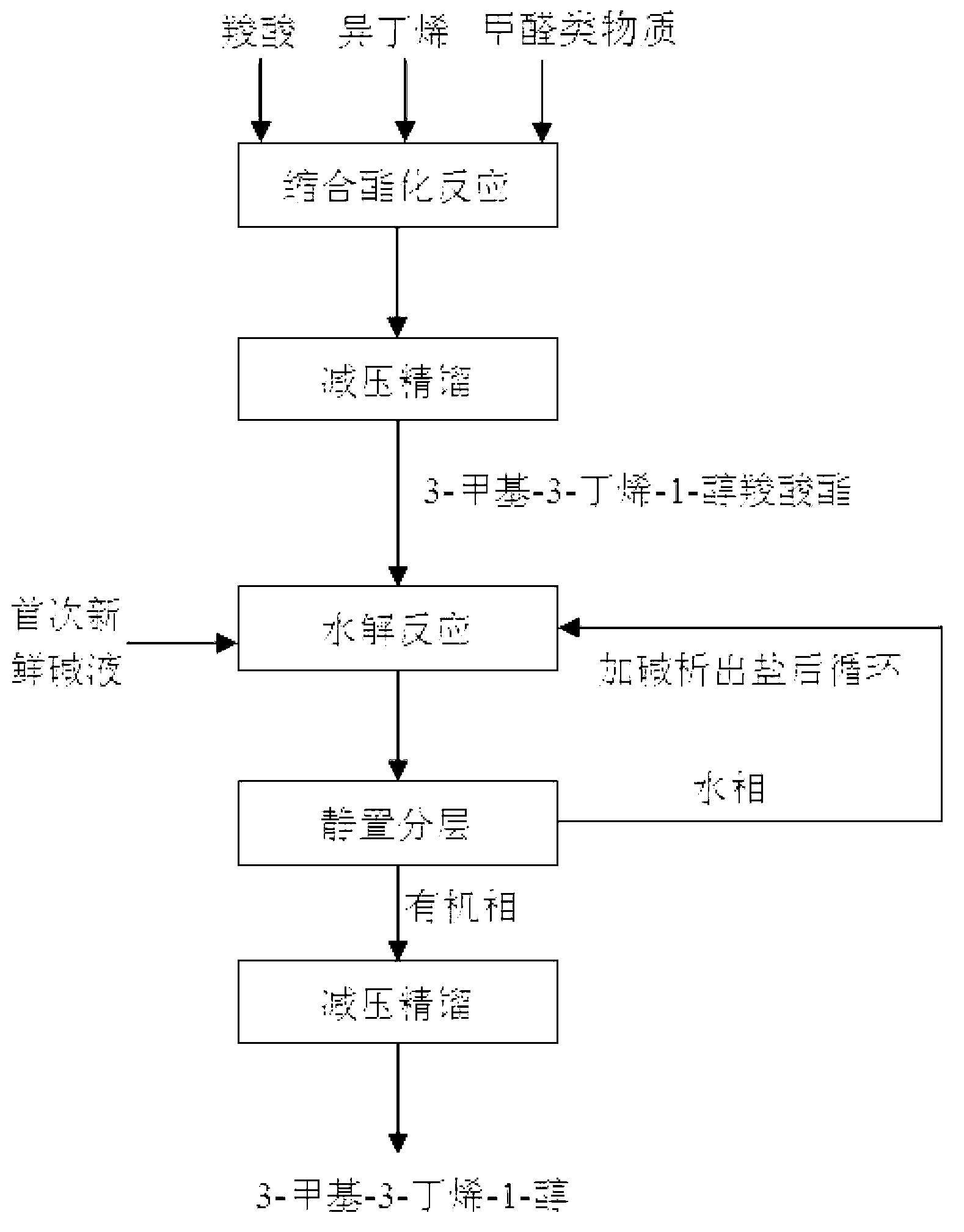
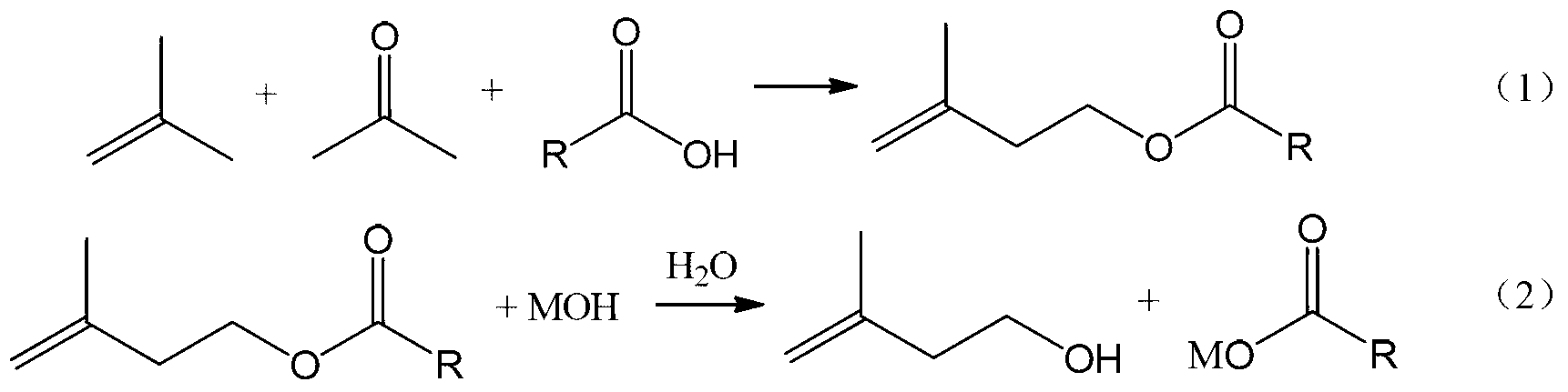
[0036] Add 805g of formic acid, 210g of paraformaldehyde and 1372g of isobutene into a 5L high-pressure stirred tank, control the reaction temperature at 130°C, and the reaction pressure at 1.5MPa. After 4 hours of reaction, 3-methyl-3-butene-1 -Alcohol formate yield is 94.6%.
[0037]The esterification reaction solution was rectified under reduced pressure to obtain 758.7 g of 3-methyl-3-buten-1-ol formate with a mass fraction of 99.5%. Add the obtained 3-methyl-3-buten-1-ol formate into a 5L stirred tank reactor, add 1942.4g of NaOH solution with a mass fraction of 15wt%, and react at 50°C for 0.8h to obtain water The phase was 2127.9g, and the weight of 3-methyl-3-buten-1-ol in the oil phase was 558.7g by internal standard method analysis, and the hydrolysis yield was 98.1%. The overall yield of 3-methyl-3-buten-1-ol was 93.0%.
Embodiment 2
[0039] Add 840g of acetic acid, 210g of paraformaldehyde and 1960g of isobutene into a 5L high-pressure stirred tank, control the reaction temperature at 120°C, and the reaction pressure at 1.2MPa. After 8 hours of reaction, 3-methyl-3-butene -1-ol acetate yield was 90.7%.
[0040] The esterification reaction solution was rectified under reduced pressure to obtain 816.8 g of 3-methyl-3-buten-1-ol acetate with a mass fraction of 99.5%. Add the obtained 3-methyl-3-buten-1-ol acetate into a 5L stirred tank reactor, add 666.6g of NaOH solution with a mass fraction of 40wt%, and react at 60°C for 0.5h to obtain water The phase is 844.4g, and the weight of 3-methyl-3-buten-1-ol in the oil phase is 526.4g by internal standard method analysis, and the hydrolysis yield is 96.4%. The overall yield of 3-methyl-3-buten-1-ol was 87.4%.
Embodiment 3
[0042] Add 2072g of propionic acid, 210g of paraformaldehyde and 1568g of isobutene into a 5L high-pressure stirred tank, control the reaction temperature at 190°C, and the reaction pressure at 4MPa. After 1 hour of reaction, 3-methyl-3-butene -1-ol propionate yield was 93.1%.
[0043] The esterification reaction solution was rectified under reduced pressure to obtain 930.1 g of 3-methyl-3-buten-1-ol propionate with a mass fraction of 99.5%. Add the obtained 3-methyl-3-buten-1-ol propionate into a 5L stirred tank reactor, add 2606.8g of NaOH solution with a mass fraction of 10wt%, and react at 100°C for 0.1h to obtain water The phase is 2971.8g, and the weight of 3-methyl-3-buten-1-ol in the oil phase is 508.3g by internal standard method analysis, and the hydrolysis yield is 90.7%. The overall yield of 3-methyl-3-buten-1-ol was 84.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com