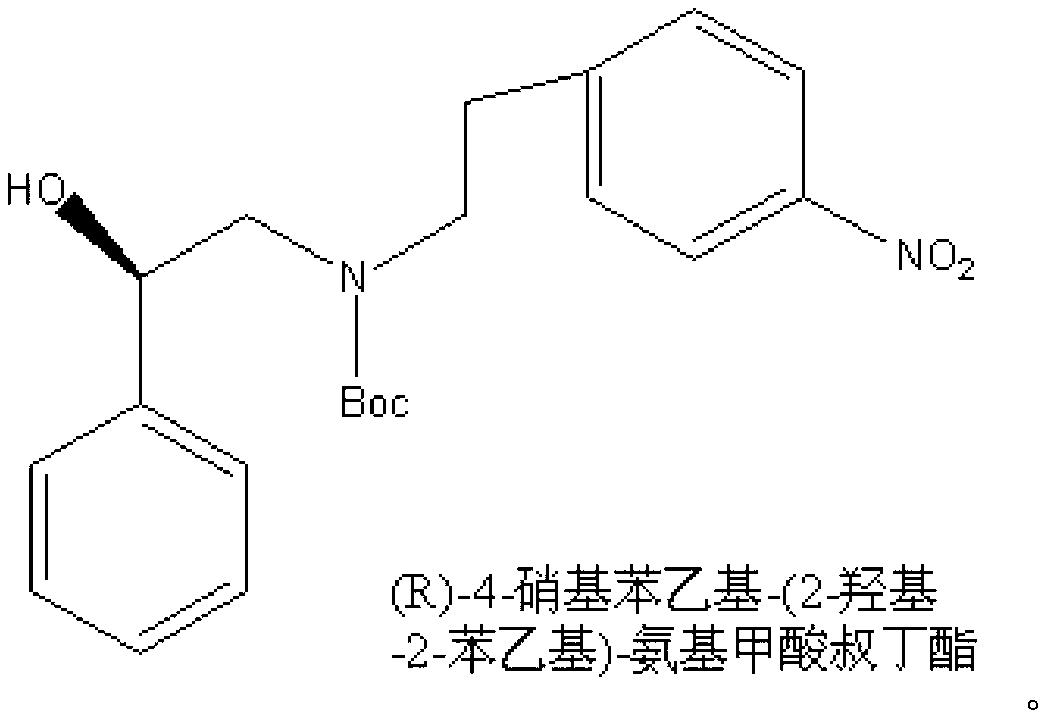
Synthesizing method of (R)-4-nitrophenethyl-(2-hydroxy-2-phenylethyl)-tert-butyl carbamate
A technology of nitrophenethyl and nitrophenethylamine is applied in the field of pharmaceutical synthesis, can solve the problems of complicated post-processing steps, high production cost, low product yield and the like, and achieves simple and easy processing, low cost, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
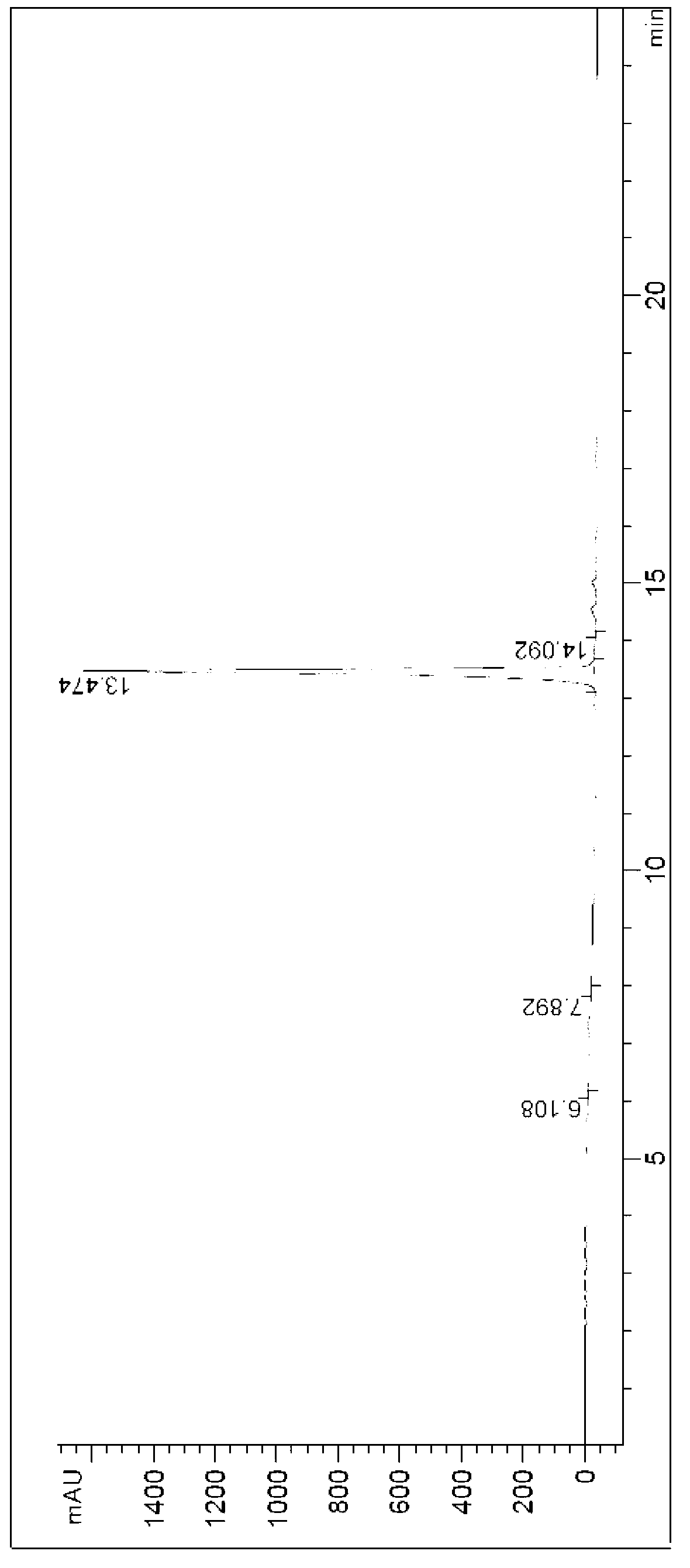
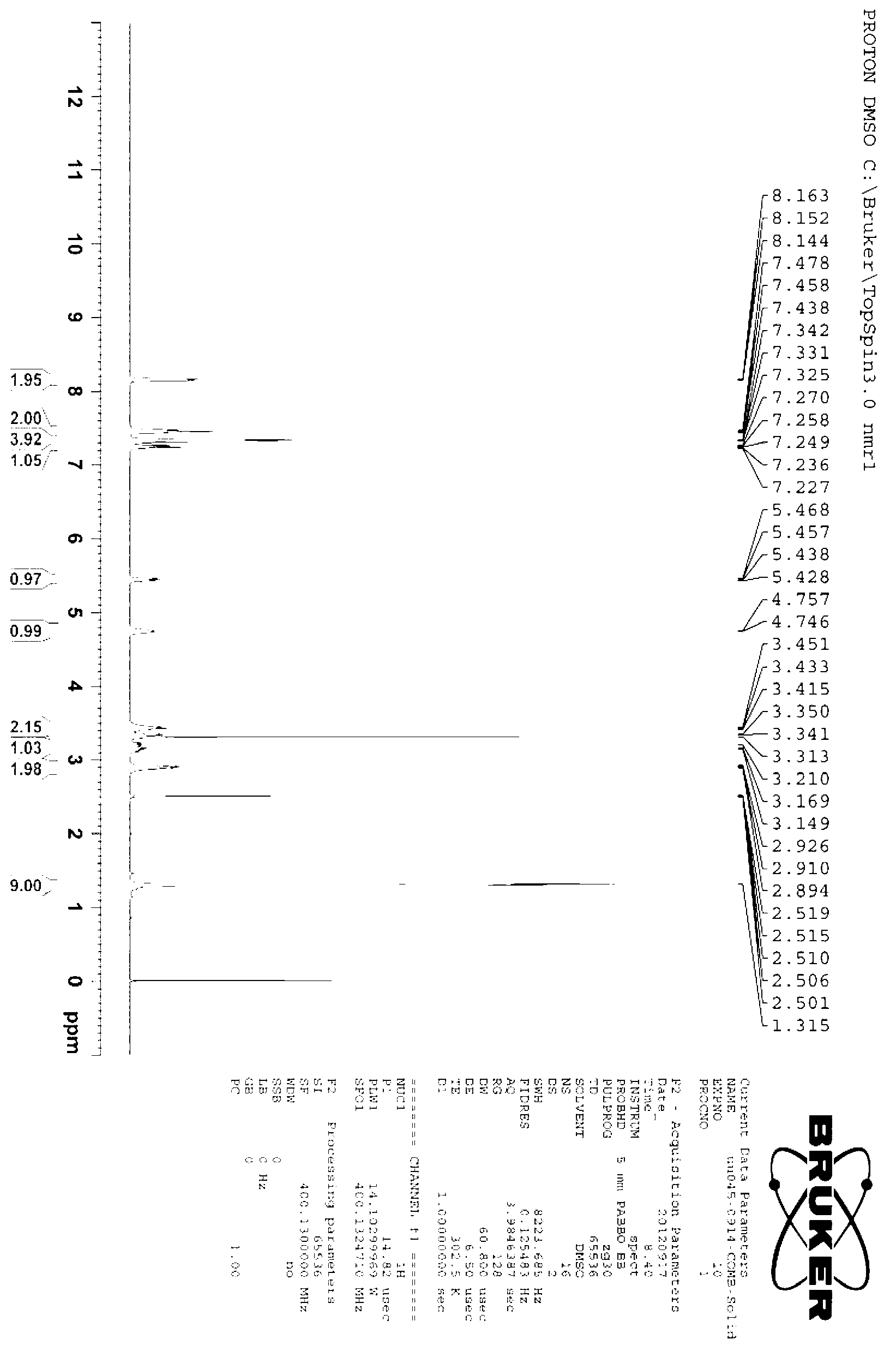
[0042] Under the protection of nitrogen, 1.67g of p-nitrophenylethanol and 7g of o-iodobenzoic acid were successively dissolved in 50mL of ethyl acetate to form a reaction solution, and the reaction solution was refluxed and stirred for 1 hour until the reaction was detected by TLC. The complete reaction solution was lowered to room temperature, and the filtrate and filter cake were obtained by suction filtration. The filter cake was washed twice with 20 mL of ethyl acetate to obtain p-nitrophenylacetaldehyde, and the filtrates were combined and concentrated for further use.
[0043]At 0°C, under the protection of nitrogen, add 1.37g (R)-2-amino-1-phenylethanol into a 100mL three-necked flask, and then drop 50mL of dichloromethane dissolved in the crude p-nitrophenylacetaldehyde Add it to a three-necked flask to form a reaction solution. Under the protection of nitrogen, the stirring was continued for 1 hour, and 0.76 g of sodium borohydride was added into the reaction soluti...
Embodiment 2
[0046] Under the protection of nitrogen, 3.34g of p-nitrophenylethanol and 10.1g of potassium permanganate were sequentially dissolved in 100mL of 1,4-dioxane to form a reaction solution, and the reaction solution was refluxed and stirred for 3 hours until the reaction was detected by TLC. The complete reaction solution was lowered to room temperature, and the filtrate and filter cake were obtained by suction filtration. The filter cake was washed twice with 40 mL of 1,4-dioxane to obtain p-nitrophenylacetaldehyde, and the filtrates were combined, concentrated and used mechanically.
[0047] At 0°C, under the protection of nitrogen, 2.7g (R)-2-amino-1-phenylethanol was added to a 250mL three-necked flask, and then 100mL tetrahydrofuran dissolved in the crude p-nitrophenylacetaldehyde was added dropwise to A reaction solution was formed in a three-necked flask. Under the protection of nitrogen, the stirring was continued for 2 hours, and 2.3 g of metallic nickel was added into...
Embodiment 3
[0050] Under the protection of nitrogen, 2.5g of p-nitrophenylethanol and 3.1g of manganese dioxide were successively dissolved in 80mL of 1,2 dichloroethane to form a reaction solution, and the reaction solution was refluxed and stirred for 2 hours until the reaction was detected by TLC. The complete reaction solution was lowered to room temperature, and the filtrate and filter cake were obtained by suction filtration. The filter cake was washed twice with 30 mL of 1,2 dichloroethane to obtain p-nitrophenylacetaldehyde, and the filtrates were combined and concentrated for further use.
[0051] At 0°C, under the protection of nitrogen, 2.05g (R)-2-amino-1-phenylethanol was added to a 250mL three-necked flask, and then 80mL of tetrahydrofuran dissolved in the crude p-nitrophenylacetaldehyde was added dropwise to A reaction solution was formed in a three-necked flask. Under the protection of nitrogen, the stirring was continued for 2 hours, and 1.03 g of sodium borohydride was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chirality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com