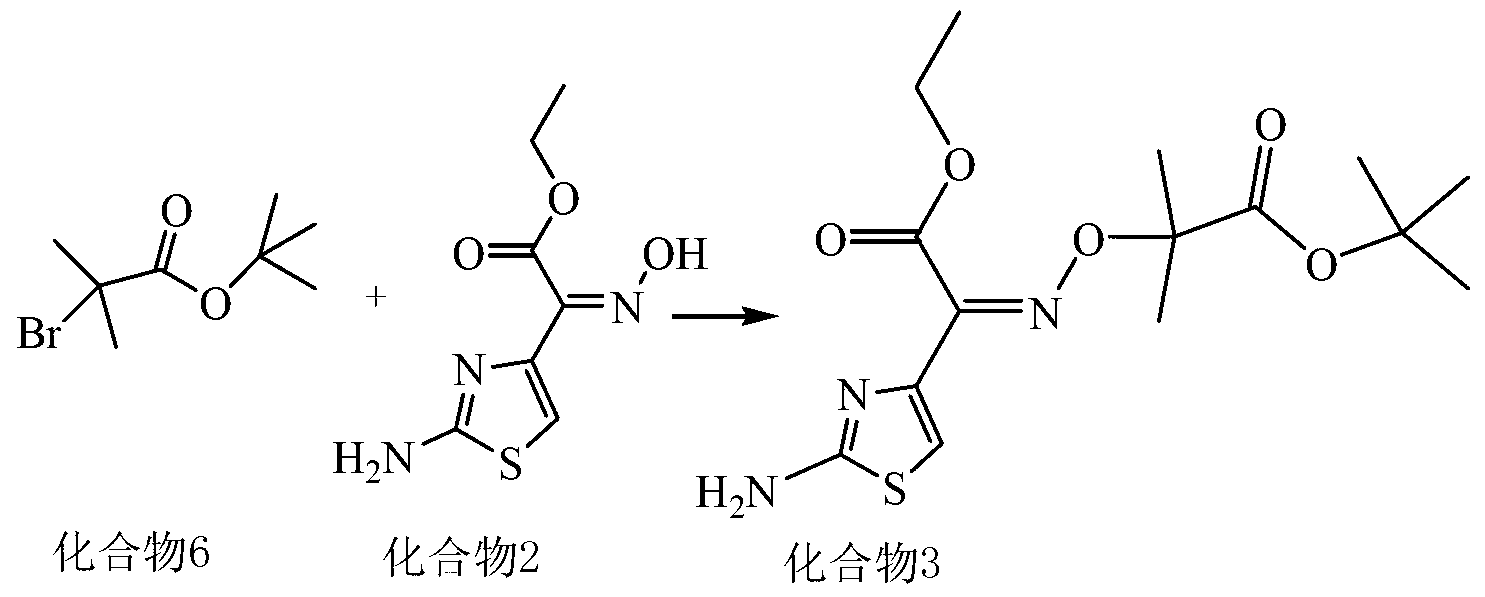
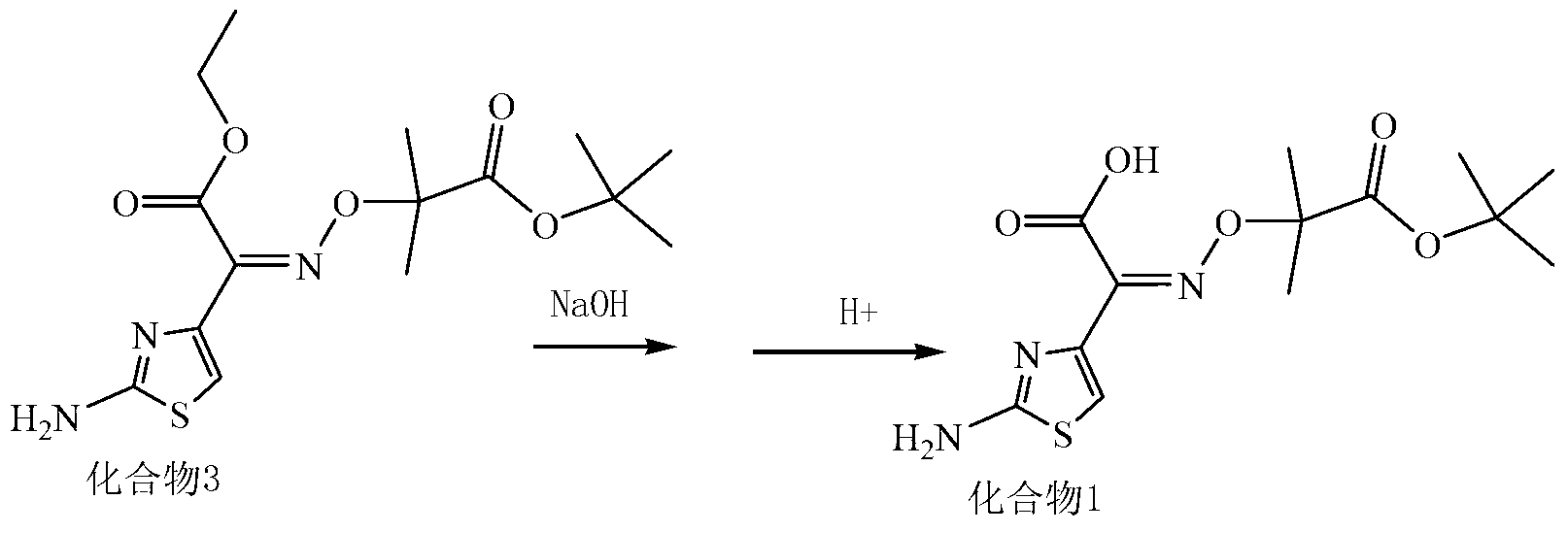
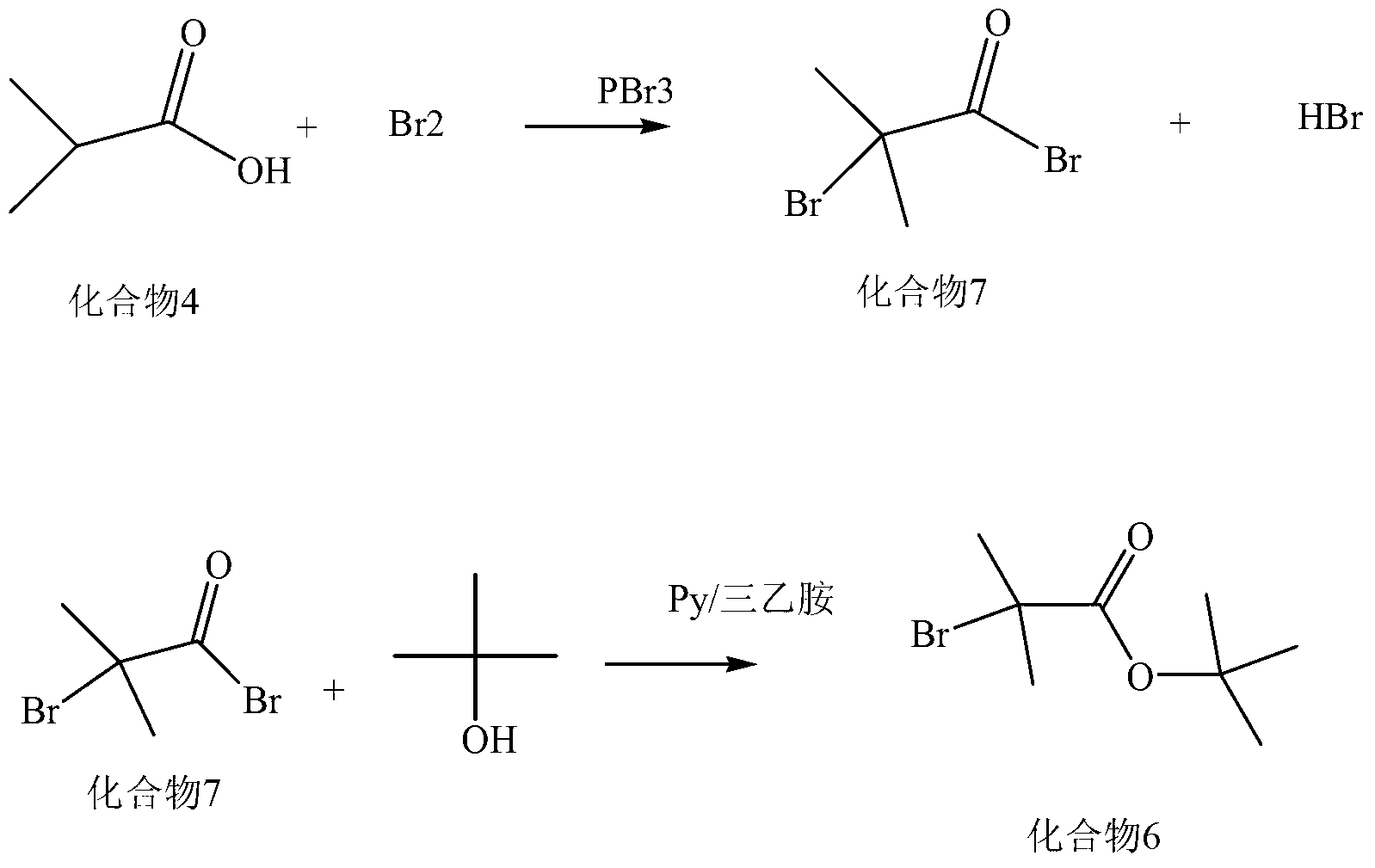
Synthesis method of new cephalosporin side-chain intermediate compound
A synthetic method and intermediate technology, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of increased difficulty in solvent recovery, serious environmental pollution, high cost, etc., and achieve high raw material utilization and conversion rate, high raw material conversion rate and yield , the effect of reducing pollution emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] 1) Preparation of α-bromoisobutyric acid
[0069] Add 88.1g (1.0mol) of isobutyric acid into a clean 500ml four-necked reaction flask, raise the temperature to 110°C, keep stirring for 10 minutes, add 40g (0.25mol) of bromine slowly for the first time, and control the dropping time for 3 hours After the dropwise addition, the reaction was kept for 10 minutes; then 18g (0.25mol) of chlorine gas was slowly introduced, and the time of introduction was controlled for 3 hours; the second time, 20g (0.125mol) of bromine was slowly added dropwise and continued for 3 hours, and the reaction was kept for 30 minutes. Minutes; then continue to introduce 8.5g (0.120mol) of chlorine gas and control the time for 1 hour; continue to drop bromine 20g (0.125mol) for the third time, control the dropping time for 3 hours, and keep the temperature at 115°C for 2-3 hours after the dropping , and then 8.8 g (0.125 mol) of chlorine gas was slowly introduced into the mixture for a controlled t...
Embodiment 2
[0077] 1) Preparation of α-bromoisobutyric acid
[0078] Add 88.1g (1.0mol) of isobutyric acid into a clean 500ml four-necked reaction flask, raise the temperature to 125°C, keep stirring for 10 minutes, add 40g (0.25mol) of bromine slowly for the first time, and control the dropping time for 3 hours , the dropwise addition is completed and the heat preservation reaction is 30 minutes; then slowly feed 0.3 mol of chlorine gas, and the time of feeding is controlled for 3 hours; the second time, 0.2 mol of bromine is slowly added dropwise, and the time of the dropwise addition is controlled for 2 hours, and the dropwise addition is completed for 10 minutes of heat preservation reaction ; Then slowly feed 0.22mol of chlorine gas, and control the feed time for 2 hours. Distill under reduced pressure (vacuum degree ≥ -0.095Mpa), collect a colorless transparent liquid after the temperature is constant, and become a white solid α-bromoisobutyric acid after cooling in the receiving bo...
Embodiment 3
[0086] 1) Preparation of α-bromoisobutyric acid
[0087] Add 88.1g (1.0mol) of isobutyric acid into a clean 500ml four-necked reaction flask, raise the temperature to 120°C, keep warm and stir for 5 minutes, add 48g (0.3mol) of bromine slowly for the first time, and control the dropping time for 3 hours , the dropwise addition is completed and the insulation reaction is 20 minutes; then the chlorine gas 21.3 (0.3mol) is slowly introduced, and the feeding time is controlled for 3 hours; the second time, bromine 20g (0.125mol) is slowly added dropwise and continued to drop for 2 hours, and the insulation reaction is 20 minutes. Minutes; then continue to introduce 8.8g (0.125mol) of chlorine gas for 1 hour; continue to add bromine 20g (0.125mol) dropwise for the third time, control the dropping time for 2 hours, and keep warm at 125°C for 2 hours after the dropping Slowly feed 8.8g (0.125mol) of chlorine gas for a controlled time of 1 hour. Distill under reduced pressure (vacuum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com