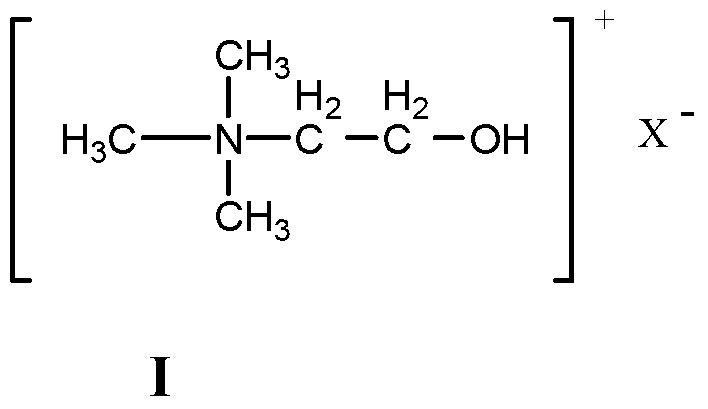
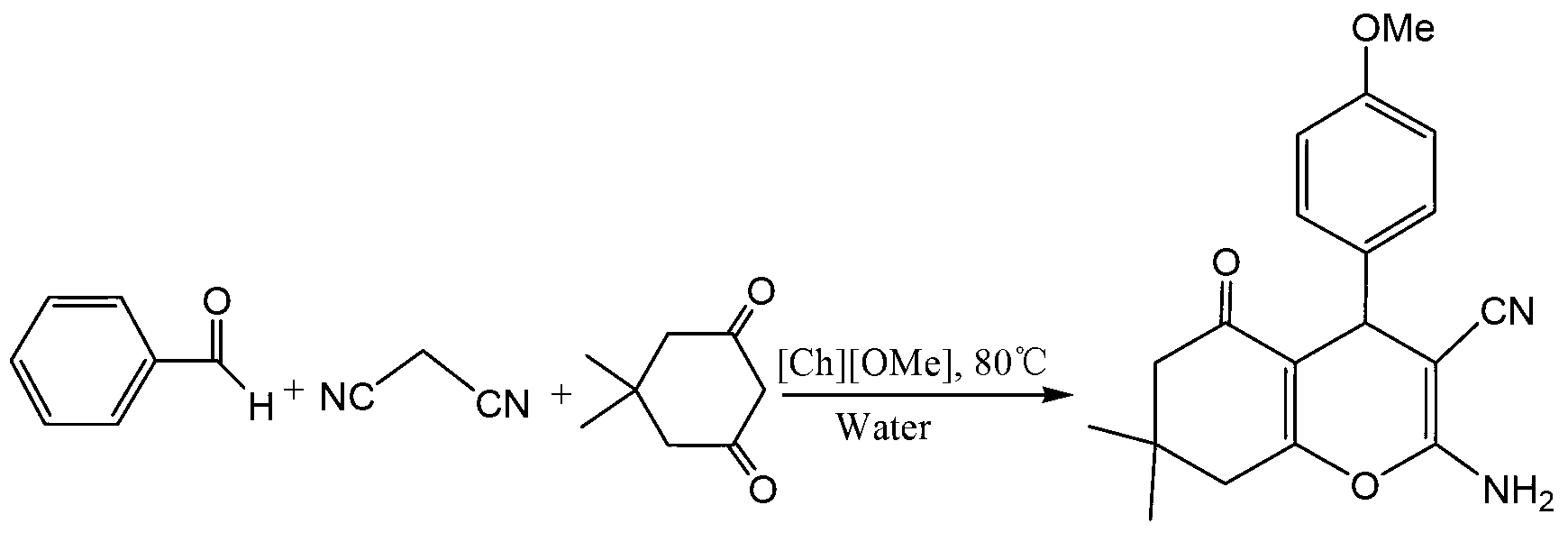
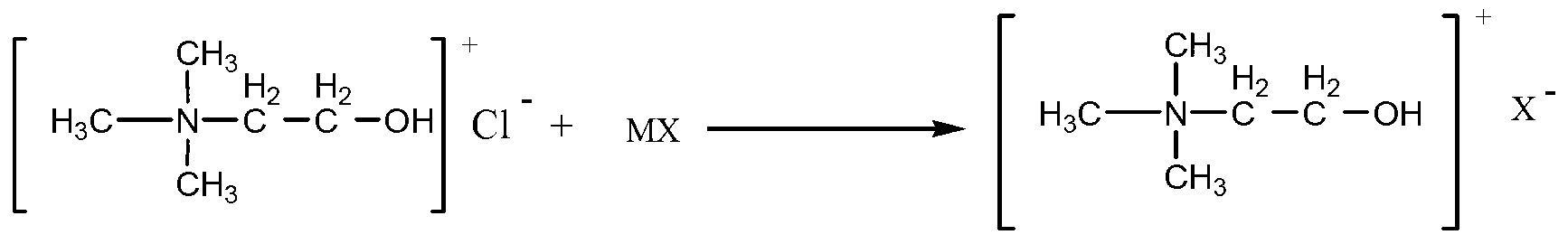Method for preparing benzopyran derivative by choline chloride functional ion liquid catalysis
A technology of benzopyran derivatives and functional ionic liquids, which is applied in the field of catalyzed preparation of benzopyran derivatives by choline chloride functional ionic liquids, can solve the problems of poor catalyst reusability, cumbersome post-treatment process, and low yield of target products. efficiency and other issues, to achieve the effect of good reusability, simple post-processing, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add benzaldehyde (5mmol), malononitrile (6mmol), dimethyl ketone (6mmol), 5mL water, 0.25mmol ionic liquid [Ch][OMe] into a 50mL single-necked bottle, stir at 80°C for 1 hour for TLC detection, the raw material disappeared, filtered, and vacuum-dried the filter cake to obtain the product with a yield of 96% and a content of 99%. Confirmation of the resulting product structure: 1 H NMR (400MHz, DMSO-d 6 )(ppm):7.29-7.10(m,5H),7.03(br,s,2H),4.15(s,1H),2.51(br,s,3H),2.25(d,1H,J=16Hz), 2.08(d,1H,J=16Hz),1.05(s,3H),0.96(s,3H); 13 C NMR (100MHz, DMSO-d 6 )(ppm): 196.3, 163.2, 159.3, 145.6, 128.9, 127.9, 127.2, 121.1, 113.4, 60.1, 50.8.
[0025]
Embodiment 2
[0027] Add p-methoxybenzaldehyde (5mmol), malononitrile (6mmol), dimethyl ketone (6mmol), 5mL water, and 0.25mmol ionic liquid [Ch][OMe] into a 50mL single-necked bottle in sequence, and stir at 80°C for 3 After 1 hour TLC detection, the raw material disappeared, filtered, and vacuum dried the filter cake to obtain the product, with a yield of 98% and a content of 98%. Confirmation of the resulting product structure: 1 H NMR (400MHz, DMSO-d 6 )(ppm):7.05(d,2H,J=8.8Hz),6.93(br,s,2H),6.78(d,2H,J=8.8Hz),4.13(s,1H),3.75(s,3H ),2.48(br,s,2H),2.25(d,1H,J=16Hz),2.06(d,1H,J=16Hz),1.01(s,3H); 13 C NMR (100MHz, DMSO-d 6 )(ppm): 196.2, 162.8, 159.1, 158.6, 137.6, 128.6, 120.5, 114.5, 113.7, 59.5, 55.8, 50.7, 41.1, 35.6, 32.5, 29.1, 27.6.
[0028]
Embodiment 3
[0030] Add p-nitrobenzaldehyde (5mmol), malononitrile (6mmol), dimethyl ketone (6mmol), 5mL water, and 0.25mmol ionic liquid [Ch][O(t-Bu)] into a 50mL single-necked bottle in sequence, Stir at 60°C for 0.5 hour TLC detection, the raw material disappeared, filter, and vacuum dry the filter cake to obtain the product, the yield was 92%, and the content was 98%. Confirmation of the resulting product structure: 1 H NMR (400MHz, DMSO-d 6 )(ppm):8.17(d,2H,J=8.8Hz),7.52(d,2H,J=8.8Hz),7.15(br,s,2H),4.32(s,1H),2.52(br,s ,2H),2.23(d,1H,J=16Hz),2.09(d,1H,J=16Hz),1.03(s,3H),0.95(s,3H); 13 C NMR (100MHz, DMSO-d 6 )(ppm): 196.4, 163.7, 159.3, 153.1, 146.8, 129.3, 124.3, 120.1, 113.1, 57.8, 50.3, 40.7, 36.6, 32.6, 28.9, 27.5.
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com