A method for preparing β-enriched decitabine precursor
A technology of decitabine and its precursor, which is applied in the field of preparation of decitabine precursor, can solve the problems of high recovery cost, lack of purification, and large amount of solvent used, and achieve stable and controllable operation and simple process , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of crude decitabine precursor
[0033] 600g of 5-azacytosine, 6.0g of ammonium sulfate, and 6L of hexamethyldisilazane were added to the 10L glass reactor successively, and the reaction was carried out at 120°C to 125°C under stirring to obtain a clear solution, and the unreacted hexamethylene was concentrated under reduced pressure. Methyldisilazane, the obtained residue was dissolved in 6L of methylene chloride, transferred to a 50L glass reactor, added 1L of trimethylsilyl trifluoromethanesulfonate at 1~5°C, and then added 2kg of 1-chloro-3 , 20L dichloromethane solution of 5-di-p-chlorobenzoyloxy-2-deoxy-D-ribose, incubated for 20h, adding an appropriate amount of saturated sodium bicarbonate solution to stop the reaction, adding 20L dichloromethane to dilute the reaction solution, purifying Washed with water twice, the organic layer was concentrated under reduced pressure to a large amount of solid precipitation, filtered and dried to obtain ...
Embodiment 2
[0034] Example 2: Preparation of crude decitabine precursor
[0035] 600g of 5-azacytosine, 6.0g of ammonium sulfate, and 6L of hexamethyldisilazane were successively added to the 10L glass reaction kettle, reacted to a clear solution at 125~130°C under stirring, and the unreacted hexamethyl was concentrated under reduced pressure. base disilazane, the obtained residue was dissolved in 6L of dichloromethane, transferred to a 50L glass reactor, 1L of trimethylsilyl trifluoromethanesulfonate was added at 5~10°C, and then 2kg of 1-chloro-3, A solution of 5-di-p-chlorobenzoyloxy-2-deoxy-D-ribose in 20L dichloromethane was incubated for 20h, an appropriate amount of saturated sodium bicarbonate solution was added to terminate the reaction, 20L dichloromethane was added to dilute the reaction solution, and water was purified After washing twice, the organic layer was concentrated under reduced pressure to precipitate a large amount of solid, which was filtered and dried to obtain 2....
Embodiment 3
[0036] Example 3: Preparation of beta-enriched decitabine precursors
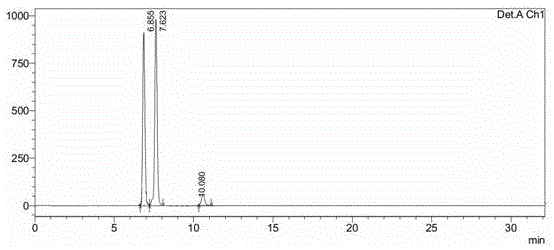
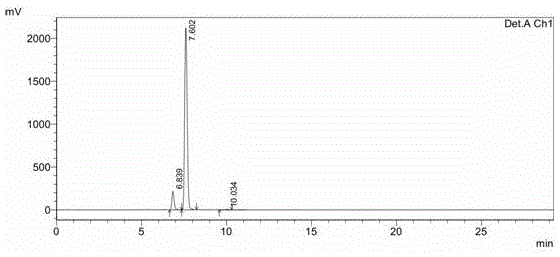
[0037] In a 20L glass reactor, add 500g of the crude decitabine precursor prepared in Example 1, add 10L of a mixed solvent of water / ethanol (V / V)=1 / 10, heat to reflux, and then stand at a temperature of about 25°C. Cool and crystallize for 12h (precipitation of α-rich body), filter, concentrate the filtrate under reduced pressure to near dryness, filter, and vacuum dry the filter cake to obtain 254g of β-enriched decitabine (α / β=1 / 11, such as figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com