Application of manganese oxide/graphene catalyst
A technology of graphene and manganese oxide, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, chemical/physical process, etc., can solve the problems of characterization of the catalytic performance of oxygen reduction reaction, and achieve easy Commercial promotion, good catalytic stability, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
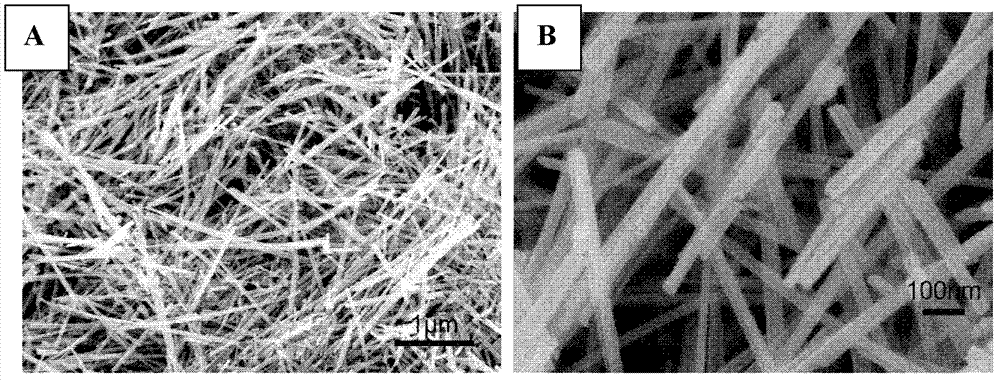
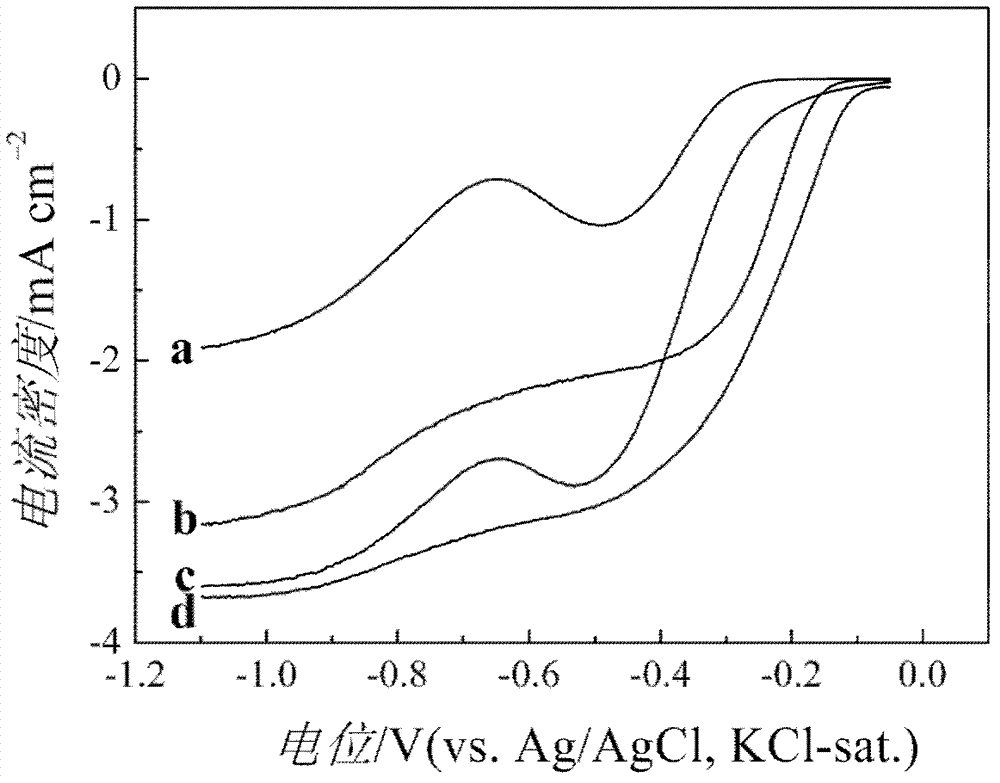
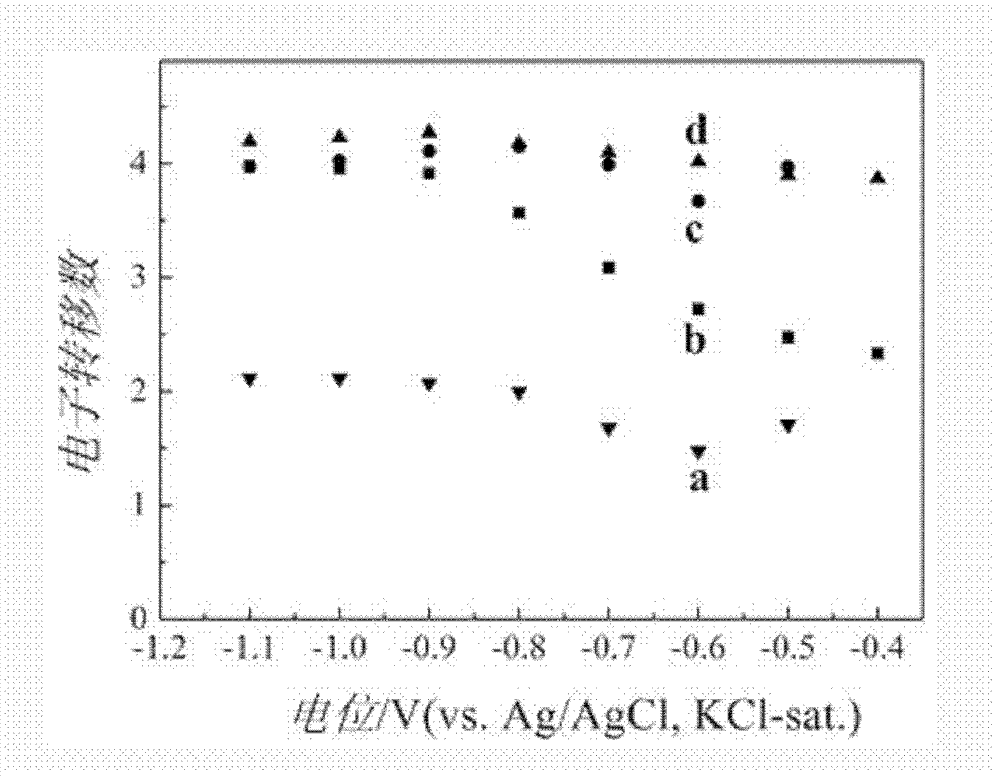
[0027] Preparation of manganese oxide / graphene catalyst: First, a layer of graphene film is modified on the surface of the working electrode by physical modification method, and then the colloidal solution of manganese oxide prepared by hydrothermal synthesis method is drip-coated on the surface of graphene film to obtain manganese oxide / Graphene Oxygen Reduction Reaction Catalyst.
[0028] The specific steps are,
[0029] Add 1g of graphite powder and 1g of sodium nitrate to 46mL of concentrated sulfuric acid, and stir in an ice bath for 4h. Then slowly add 6 g of potassium permanganate under stirring (ensure that the temperature is maintained below 10° C.). Then it was transferred to a water bath at 35°C and stirred for 2 hours, then 92 mL of ultrapure water was added, and the temperature was adjusted to 98°C and stirred for 2 hours in a water bath. At this time, the solution was bright yellow. Finally, 200 mL of warm water and 20 mL of 30% hydrogen peroxide were added, s...
Embodiment 2
[0038] The difference from Example 1 is:
[0039] The concrete steps of preparing manganese oxide / graphene oxygen reduction reaction catalyst are,
[0040] Add 1g of graphite powder and 1g of sodium nitrate to 23mL of concentrated sulfuric acid, and stir in an ice bath for 4h. Then slowly add 3 g of potassium permanganate under stirring (make sure the temperature is maintained below 10° C.). Then it was transferred to a water bath at 30°C and stirred for 3 hours, then 92 mL of ultrapure water was added, and the temperature was adjusted to 95°C and stirred for 3 hours in a water bath. At this time, the solution was bright yellow. Finally, 300 mL of warm water and 30 mL of 30% hydrogen peroxide were added, stirred for 1 h, and the solution turned brown at this time.
[0041] The obtained colloidal solution is centrifuged, then washed once with hydrochloric acid solution with a mass concentration of 25%, washed three times with ultrapure water, and finally washed once with abso...
Embodiment 3
[0048] The difference with embodiment 1 and 2 is:
[0049] The concrete steps of preparing manganese oxide / graphene oxygen reduction reaction catalyst are,
[0050] Add 1g of graphite powder and 1g of sodium nitrate to 23mL of concentrated sulfuric acid, and stir in an ice bath for 4h. Then slowly add 6 g of potassium permanganate under stirring (ensure that the temperature is maintained below 10° C.). Then it was transferred to a water bath at 40°C and stirred for 1.5 hours, then 100 mL of ultrapure water was added, and the temperature was adjusted to 93°C for 4 hours. At this time, the solution was bright yellow. Finally, 150 mL of warm water and 25 mL of 60% hydrogen peroxide were added, stirred for 1 h, and the solution turned brown at this time.
[0051] The obtained colloidal solution is centrifuged, then washed once with hydrochloric acid solution with a mass concentration of 20%, washed three times with ultrapure water, and finally washed once with absolute ethanol t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com