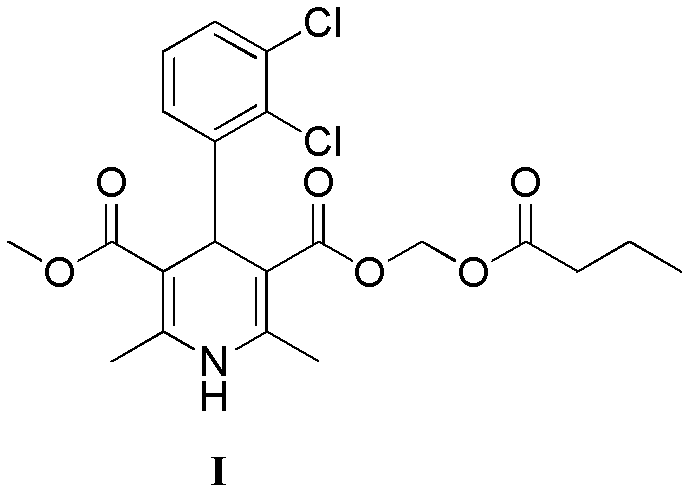
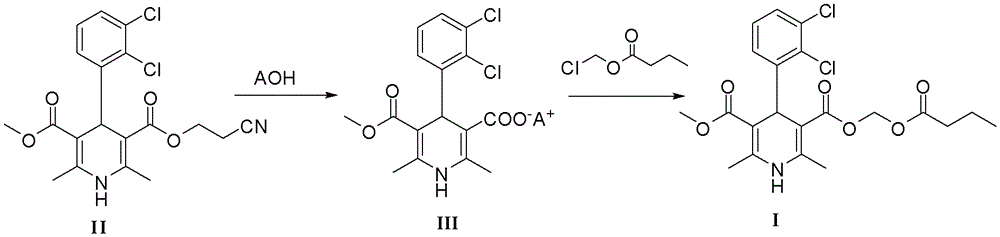
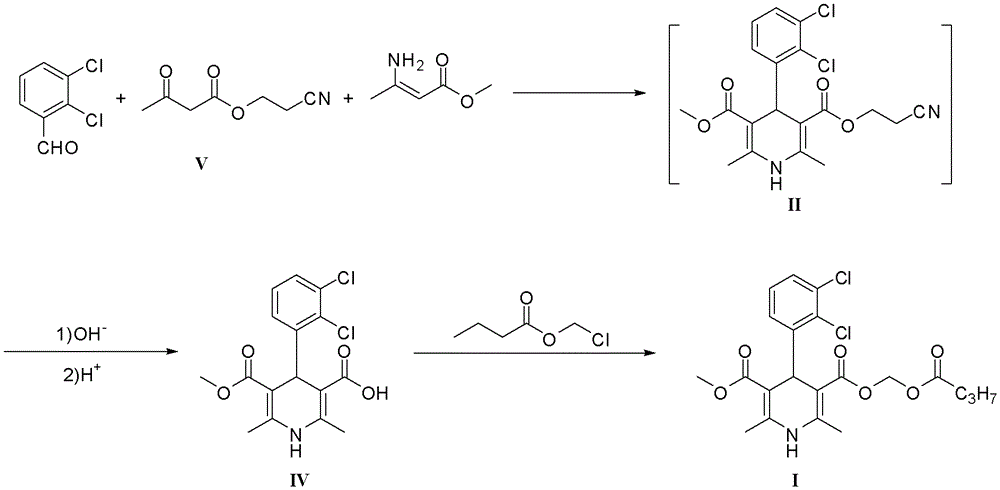
The preparation method of dihydropyridine compound
A technology of dihydro, pyridine carboxylic acid, applied in the field of preparation of dihydropyridine compounds, can solve the problems of high flammability, volatile, highly toxic acrylonitrile, etc., and achieves low cost, little environmental pollution and convenient operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of 4-(-2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-5-methoxycarbonyl-3-pyridinecarboxylic acid (IV)
[0047]
[0048] tert-butyl 3-aminocrotonate (3) (235mg, 1.5mmol) and methyl 2,3-dichlorobenzylidene acetoacetate (2) (273mg, 1mmol) were added to methanol (20ml) and reacted at 60°C 8 hours, cooled, spin-dried, and separated by column chromatography (ethyl acetate:petroleum ether=1:3) to obtain 4-(-2,3-dichlorophenyl)-1,4-dihydro-2,6- Dimethyl-3,5-pyridinedicarboxylic acid-(tert-butyl)methyl ester (1) 298 mg, yield 72.5% (based on 2).
[0049] m / z: 412(M+H); 1 HNMR (CDCl 3 , ppm): δ=7.02-7.37 (m, 3H), 4.78 (s, 1H), 3.77 (s, 3H), 2.24 (s, 3H), 2.22 (s, 3H), 1.39 (s, 6H).
[0050] 4-(-2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic acid-(tert-butyl)methyl ester (1) (412mg, 1mmol ) was dissolved in dichloromethane (10ml), added trifluoroacetic acid (1.5mmol), stirred at 20°C for 2.5h, spin-dried the solvent, added ethyl acetate and...
Embodiment 2
[0053] Preparation of 4-(-2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-5-methoxycarbonyl-3-pyridinecarboxylic acid (IV)
[0054]
[0055]3-Aminocrotonate-(1,1-dimethyl)benzyl ester (5) (26.3g, 0.12mol) and 2,3-dichlorobenzylidene acetoacetate (2) (27.3g, 0.1 mol) was added in isopropanol (300ml), reacted at 80°C for 9 hours, cooled, precipitated crystals, filtered, and dried to obtain 33.3g of product (4), with a yield of 70.3% (based on 2).
[0056] m / z: 474(M+H); 1 HNMR (CDCl 3 , ppm): δ=7.60(m, 1H), 7.38-7.51(m, 5H), 7.02-7.15(t, 3H), 4.78(s, 1H), 3.77(s, 3H), 2.26(s, 6H ), 1.49(s, 6H).
[0057] 4-(-2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic acid-((1,1-dimethyl)benzyl) Methyl ester (4) (4.74g, 0.01mol) was added to ethyl acetate (50ml), cooled to -10°C, and 1N hydrochloric acid-ethyl acetate solution (0.01mol) was added dropwise under stirring, and the drop was completed, stirred for 1h, filtered , the filter cake was washed with cold ethy...
Embodiment 3
[0059] Preparation of 4-(-2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-5-methoxycarbonyl-3-pyridinecarboxylic acid (IV)
[0060]
[0061] Benzyl 3-aminocrotonate (7) (0.2 g, 1.05 mmol) and methyl 2,3-dichlorobenzylidene acetoacetate (2) (0.2 g, 0.7 mmol) were added to methanol (10 ml), React at 60°C for 3 hours, cool in an ice-water bath, precipitate crystals, filter, wash with a small amount of cold methanol, and dry to obtain 0.27 g of product (6), with a yield of 86.3% (based on 2).
[0062] m / z: 446(M+H); 1 HNMR (CDCl 3 , ppm): δ=7.31(m, 1H), 7.06-7.21(m, 5H), 7.02-7.19(t, 3H), 5.6(s, 1H), 5.52(s, 3H), 5.07-5.14(s , 3H), 2.34(s, 6H).
[0063] 4-(-2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic acid-(benzyl)methyl ester (6) (446g, 1mol ) into methanol (3L), then 10% by weight of Pa / C (4.46g), react with hydrogen at 30°C for 8 hours, filter off Pd / C, concentrate, precipitate solids, filter, filter the cake with an appropriate amount of cold metha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com