2, 5-diaryl-1, 3, 4-oxadiazole fluorescent molecule and preparation method thereof
A fluorescent molecule, oxadiazole technology, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., to achieve the effect of excellent fluorescent emission performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
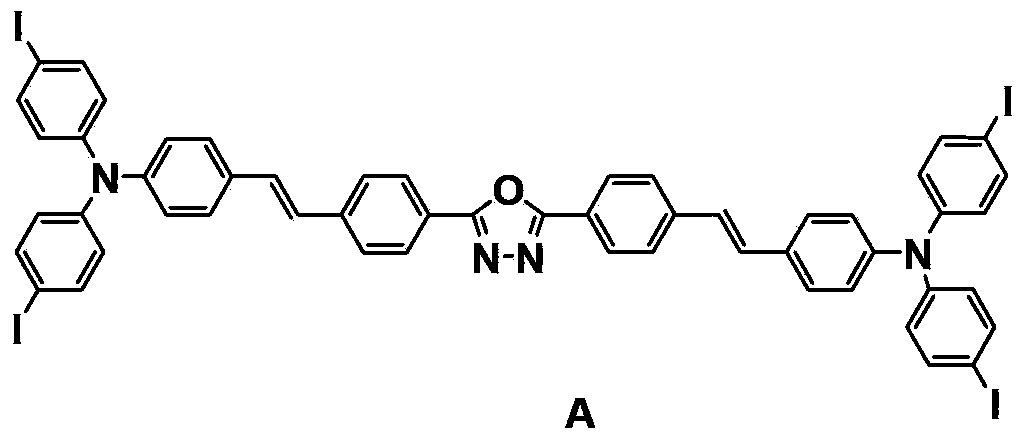
[0014] Embodiment 1 prepares compound A
[0015] Dissolve ethyl [1,3,4]-oxadiazole-2,5-dibenzyl phosphate (1.0g, 1.91mmol) in 15mL DMF, under nitrogen protection, slowly add potassium tert-butoxide in ethanol dropwise under full stirring Solution (mass fraction is 30%, 5.65mmol), stirred and reacted for 0.5h. Added 4-[N,N-bis(4-iodophenyl)]benzaldehyde (2.0g, 3.83mmol), heated to 85°C, refluxed 8h. The reaction solution was poured into 50mL of ethanol, a large amount of flocculent precipitates were formed, stood still, and filtered with suction. The obtained solid was washed with hot ethanol several times to obtain 1.5g of yellow-green solid -2,5-bis{4-{4-[ N,N-bis(4-iodophenyl)amino]styrene}phenyl}-[1,3,4]-oxadiazole (A), yield 63%.
[0016] Analysis of the obtained compound A:
[0017] 1 H NMR (300MHz, CDCl 3 )δ:8.16(d,J=8.22Hz,4H),7.68(d,J=J=8.19Hz,4H),7.59(d,J=8.79Hz,8H),7.47(d,J=8.61Hz, 4H),7.24(d,J=18.72Hz,4H),7.08(d,J=8.67Hz,4H),6.89(d,J=8.82,8H)
[0018] The stru...
Embodiment 2
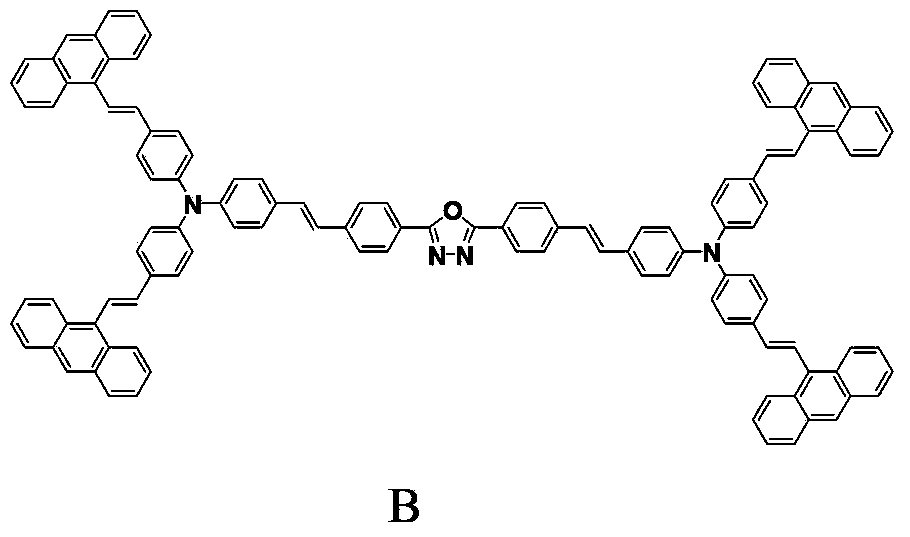
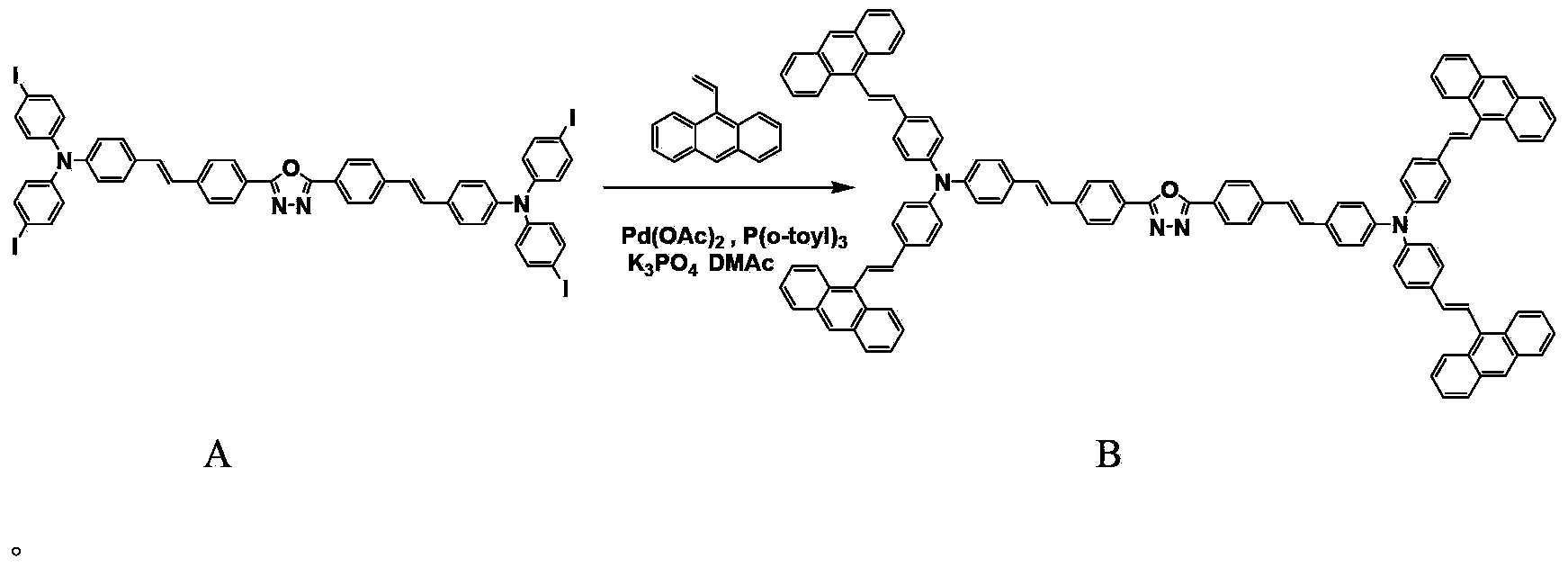
[0021] Embodiment 2 prepares compound B
[0022] Under the protection of anhydrous, oxygen and nitrogen, add 2,5-bis{4-{4-[N,N-bis(4-iodophenyl)amino]styrene}phenyl}- [1,3,4]-Oxadiazole (A) 0.316g (0.25mmol), 9-vinyl anthracene (purchased from Bailingwei Chemical Co., Ltd.) 0.245g (1.20mmol) and palladium acetate (purchased from Bailingwei Chemical Co., Ltd. Co., Ltd.) 0.020g (0.09mmol), tris(o-tolyl)phosphine (purchased from Bailingwei Chemical Co., Ltd.) 0.057g (0.18mmol), potassium phosphate 0.298g (1.40mmol) and 6.00mL of anhydrous DMAc, oil bath Heat to 140°C and reflux for 72 hours. After the reaction, filter to remove impurities, wash with dichloromethane solution, slowly add the solution dropwise to methanol solution, and a yellow solid precipitates. After drying, the product is purified by column chromatography and gradient elution , a light yellow solid was obtained with a yield of 30.8%. m.p.246-247°C.
[0023]
[0024] Analysis of the resulting compound B:
...
Embodiment 3
[0029] The fluorescence detection of embodiment 3 compound B
[0030] Example compound B has excellent fluorescence emission properties, and there is a strong intramolecular charge transfer in the conjugated system, which can be used as a two-photon absorption and fluorescence material. In THF solution, the maximum wavelength of its ultraviolet absorption is 410nm, the maximum wavelength of linear fluorescence is at 519nm, and the fluorescence lifetime is 1.48ns. Excited with 800nm femtosecond laser, the two-photon absorption cross-section is measured as 915GM by two-photon induced fluorescence method, and the two-photon fluorescence wavelength is located at 523nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com