Conjugated organic small molecule as well as preparation method and application thereof
A small molecule, organic technology, applied in the field of conjugated organic small molecules and their preparation, can solve the problems of backward development of small molecule donor materials, and achieve the effect of realizing electron mobility, improving photovoltaic performance, and improving miscibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
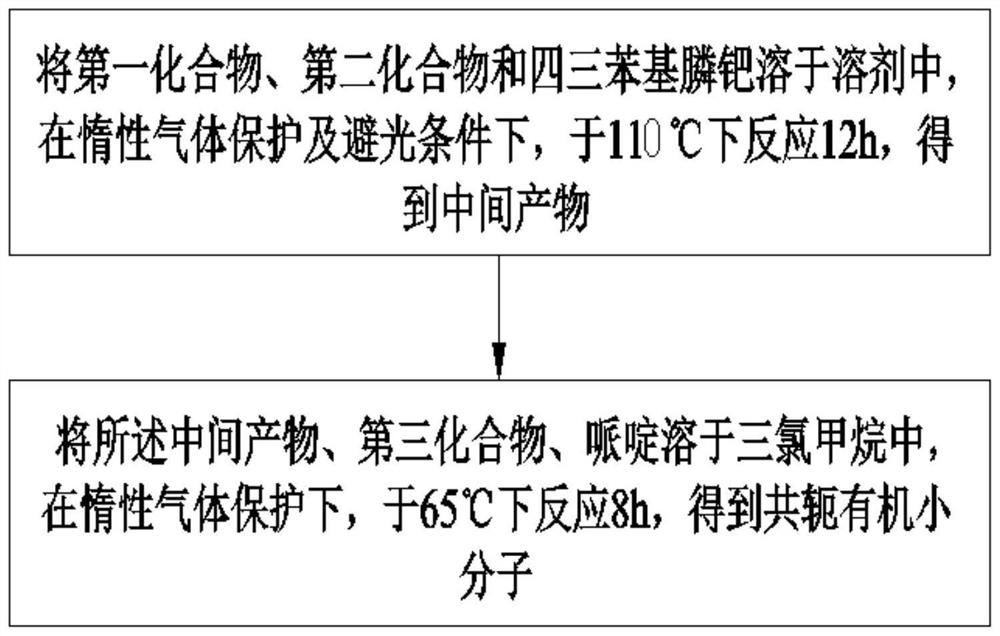
[0058] see figure 1 As shown, another embodiment of the present invention provides a method for preparing a conjugated organic small molecule, comprising:
[0059] Dissolve the first compound, the second compound and tetrakistriphenylphosphine palladium in a solvent, and react at 110° C. for 12 h under inert gas protection and dark conditions to obtain an intermediate product;
[0060] Dissolving the intermediate product, the third compound, and piperidine in chloroform, and reacting at 65° C. for 8 hours under the protection of inert gas to obtain a conjugated organic small molecule;
[0061] Wherein, the structural formula of the first compound is:
[0062]
[0063] The structural formula of the second compound is:
[0064]
[0065] The structural formula of the third compound is:
[0066]
[0067] In the specific embodiment, the first compound, the second compound and tetrakistriphenylphosphine palladium are added to the reaction vessel, and then toluene is used...
Embodiment 1
[0081] The purpose of this embodiment is to prepare a conjugated organic small molecule, comprising the following steps:
[0082] In a 100 mL three-necked flask, compound 1 (454 mg, 0.5 mmol), compound 2 (622 mg, 1.25 mmol), tetrakistriphenylphosphonium palladium (Pd(PPh) were added in turn 3 ) 4 , 58 mg, 0.05 mmol), and then 30 mL of toluene (Toluene) was used as a solvent, under argon protection, and heated at 110° C. and refluxed for about 12 hours in the dark. The reaction solution after the reaction was poured into water, extracted with chloroform, washed with deionized water, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to obtain a crude product. The crude product was obtained by column chromatography (petroleum ether: chloroform=1:2) to obtain a black solid P1 (472.2 mg) in a yield of ~67%. The obtained P1 (191 mg, 0.135 mmol), compound 3 (272 mg, 1.35 mmol), 20 mL of chloroform (CHCl) 3 ) and a few drops of piperidine (pipe...
Embodiment 2
[0086] The purpose of this embodiment is to prepare a conjugated organic small molecule, comprising the following steps:
[0087] P1 (283 mg, 0.2 mmol) obtained in Example 1, compound 4 (435 mg, 2.0 mmol), 30 mL of chloroform and a few drops of piperidine were successively added to a 100 mL three-necked round-bottomed flask, and heated under argon protection. Reflux at 65°C for about 8 hours. The reaction solution after the reaction was concentrated under reduced pressure, was deposited in a large amount of methanol, and the obtained solid crude product was subjected to column chromatography (petroleum ether: chloroform=1.5:1) to obtain black solid BDF-2 (313 mg), The yield was 86%.
[0088] The synthetic route of the preparation method of the conjugated organic small molecule BDF-2 described in this example is as follows:
[0089]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com