Ferrocenyl pyrimidine pincer ligand and preparation method thereof
A ferrocenyl pyrimidine clamp and base pyrimidine clamp technology are applied in the synthesis of such compounds and in the field of ferrocenyl pyrimidine clamp ligands to achieve the effects of good stability, wide substrate range and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
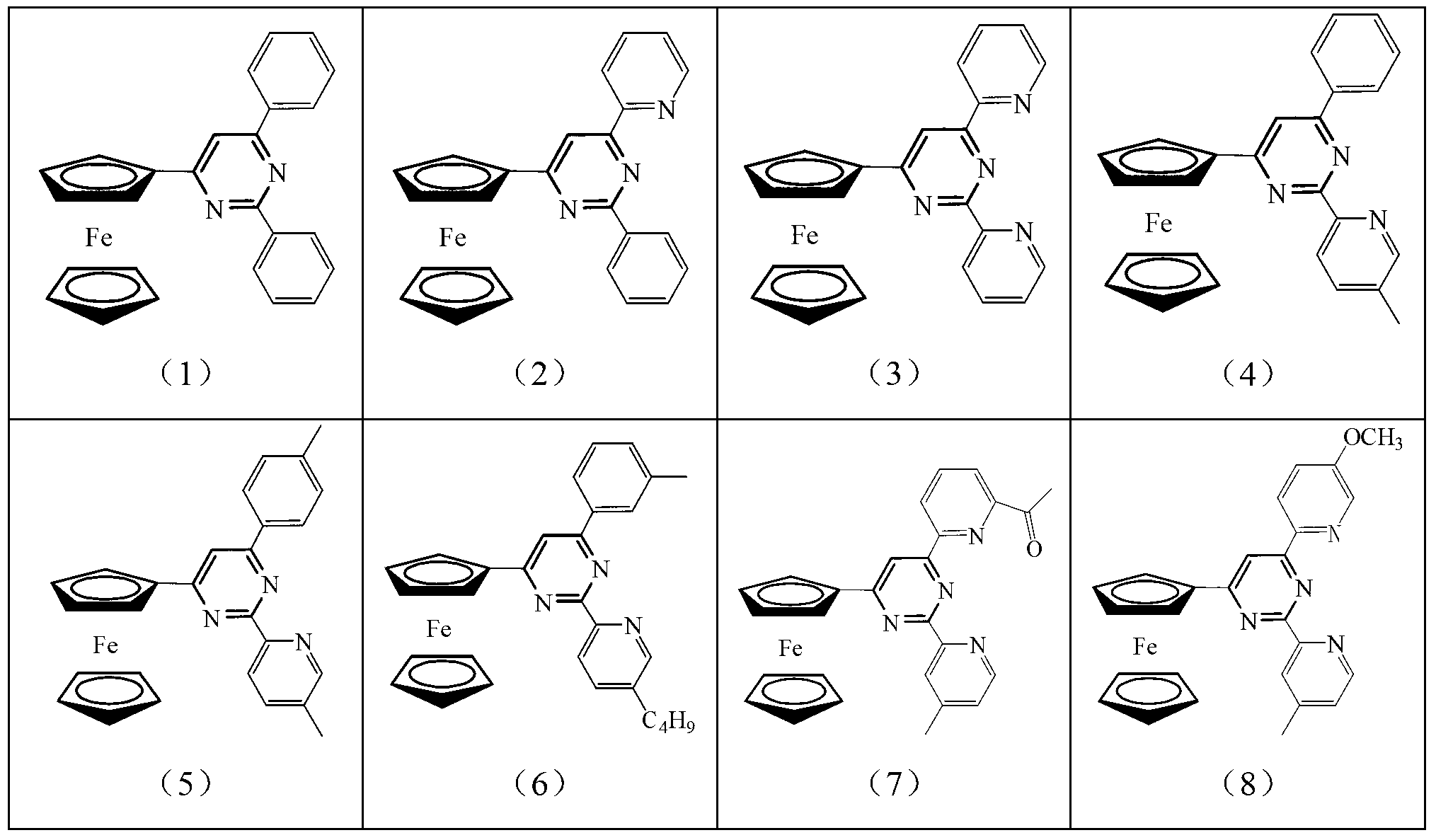
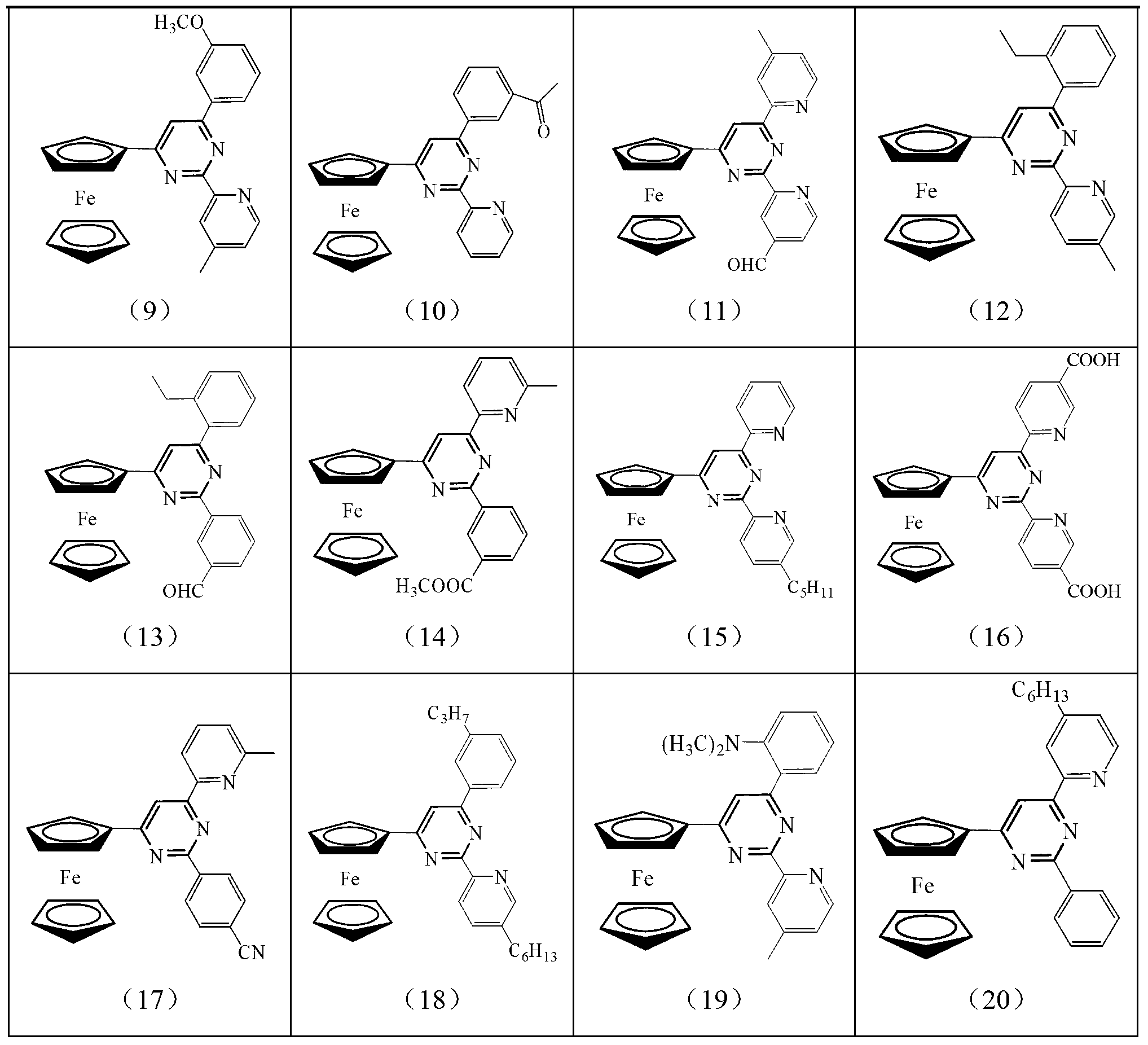
[0020] The ferrocenyl pyrimidine pincer ligand has the following general formula: Its specific structure can be:
[0021]
[0022]
Embodiment 2
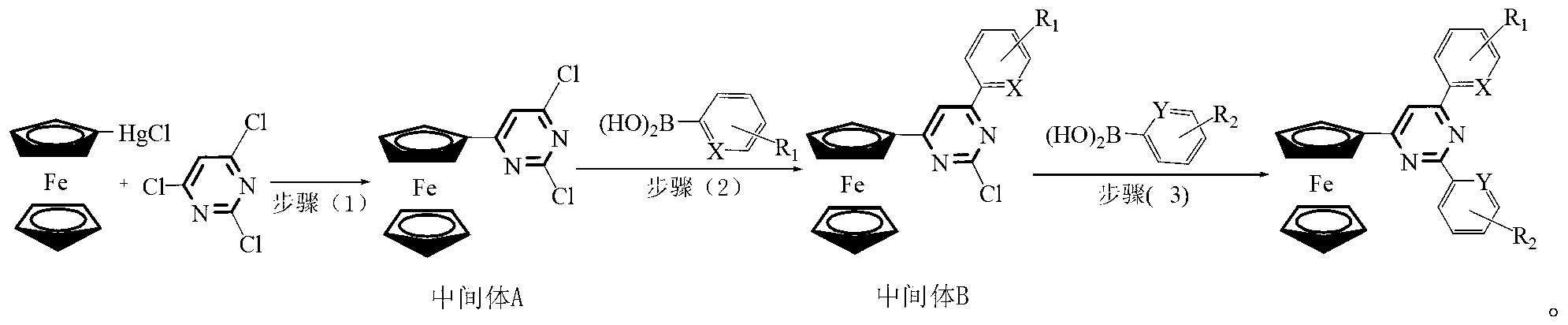
[0024] The preparation method of ferrocenyl pyrimidine pincer ligand, the steps include:
[0025]
[0026] Wherein, the possible structure of intermediate B and its corresponding product number are as follows:
[0027]
[0028]
[0029]
[0030] The preparation process of the specific product is described in detail below.
[0031] 2.1 Preparation method of ferrocenyl pyrimidine pincer ligand (1)
[0032] Step (1): Synthesis of Intermediate A
[0033] Under the protection of high-purity nitrogen, add 0.5mmol of 2,4,6-trichloropyrimidine, 0.6mmol of ferrocene mercuric chloride, and 0.005mmol of tetrakis(triphenylphosphine)palladium to a 10ml Schlek reaction tube, and replace the reaction with nitrogen Tube 3 times, and inject 5ml of tetrahydrofuran solvent with a syringe under the continuous protection of slight positive pressure nitrogen, then heat to 80°C with an oil bath under magnetic stirring, and reflux for 10 hours.
[0034] Remove the oil bath, and lower t...
Embodiment 3 2
[0117] Embodiment 3 The purposes of ferrocenyl pyrimidine pincer ligand
[0118] Synthesis of palladium catalyst—compound (21) using ferrocenyl pyrimidine pincer ligand (5):
[0119]
[0120] Under the protection of nitrogen, add 1 mmol of ferrocenyl pyrimidine pincer ligand (5), 1.2 mmol of PdCl2 and 10 ml of acetic acid into a 10 ml Schlek reaction tube, replace the reaction tube with nitrogen for 3 times, and then use an oil bath under magnetic stirring Heated to 120°C and refluxed for 24 hours. The resulting red solid was filtered off and dried to obtain the ferrocenyl pincer-shaped palladium compound (21) with a yield of 82%. The nuclear magnetic analysis data of this product are as follows: 1 H NMR. (400MHz, CDCl 3 ):δ8.71(s,1H,PyH),7.78(d,1H,PyH),7.55(d,1H,PyH),7.51(s,1H,PyH),7.47(d,1H,ArH),7.26 (d,1H,ArH),7.20(d,1H,ArH),5.13(s,2H,C 5 h 4 ),4.56(s,2H,C 5 h 4 ),4.11(s,5H,C 5 h 5 ),2.37(s,3H,CH 3 ),2.32(s,3H,CH 3 ).
[0121] Compound (21) can be used as a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com