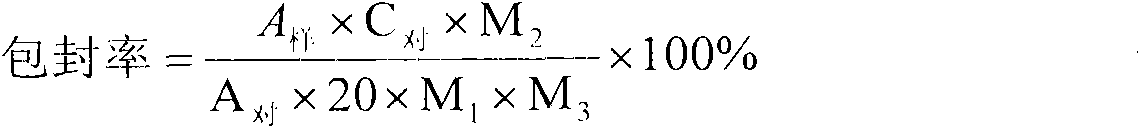Voriconazole freeze-dried powder injection for injection and preparation method thereof
A technology for freeze-dried powder injection and voriconazole, which is applied in the field of voriconazole freeze-dried powder for injection and its preparation, can solve the problems of easy precipitation, unstable dilution, poor stability and the like, and achieves good solubility, improved solubility and stability High performance and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1 (specification 50mg)
[0059] The weight of each component in the prescription of preparing 1000 voriconazole freeze-dried powder injections for injection is as follows:
[0060] Voriconazole 50g Hydroxypropyl-β-cyclodextrin 400g
[0061] Mannitol 200g 2M hydrochloric acid 250ml
[0062] 2M NaOH to adjust the pH to 5.5 and water for injection to 5L
[0063] The preparation process is as follows:
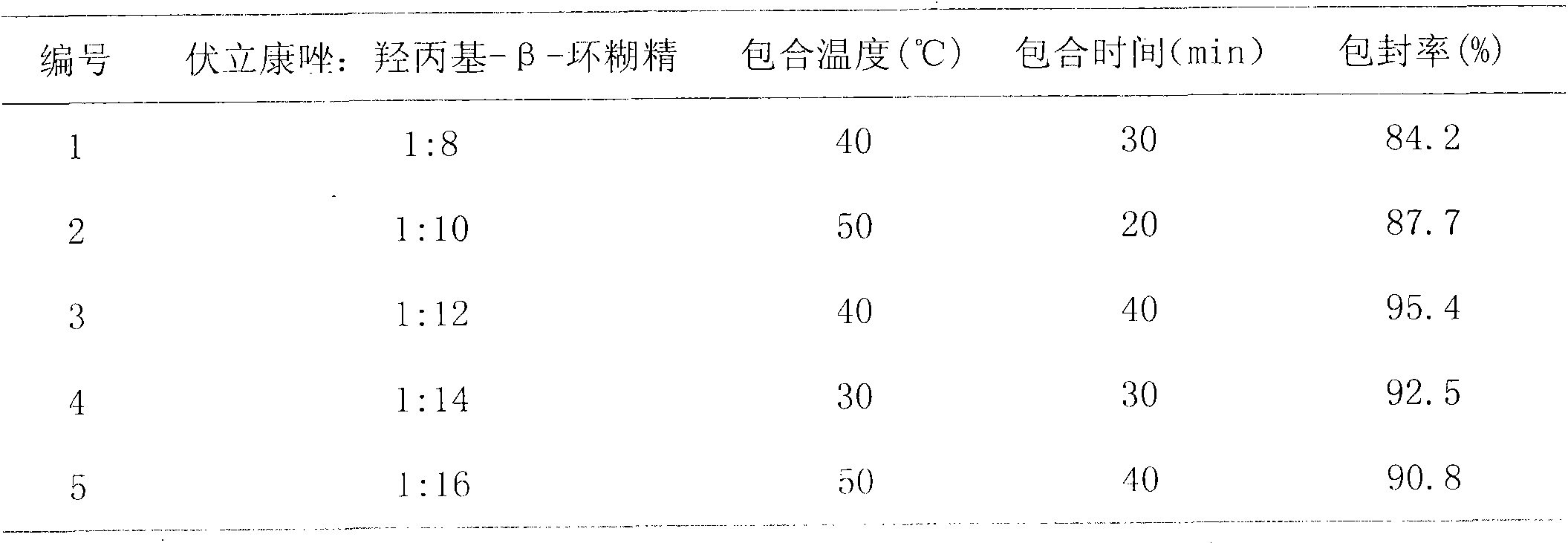
[0064] Add 400g of hydroxypropyl-β-cyclodextrin to water for injection of 30% of the prescription amount, stir to completely dissolve the hydroxypropyl-β-cyclodextrin; accurately weigh 50g of voriconazole, dissolve it with 250mL of 2M hydrochloric acid, and control Warm at 40°C, add it into the dissolved hydroxypropyl-β-cyclodextrin aqueous solution, and stir while adding; continue to stir for 30 minutes to make voriconazole inclusion as complete as possible; accurately weigh 200g of mannitol, add the above-mentioned package Stir to dissolve the mixed solution; ...
Embodiment 2
[0065] Embodiment 2 (specification 50mg)
[0066] The weight of each component in the prescription of preparing 1000 voriconazole freeze-dried powder injections for injection is as follows:
[0067] Voriconazole 50g Hydroxypropyl-β-cyclodextrin 500g
[0068] Mannitol 200g 2M hydrochloric acid 250ml
[0069] 2M NaOH to adjust the pH to 5.5 and water for injection to 5L
[0070] The preparation process is as follows:
[0071] Add 500g of hydroxypropyl-β-cyclodextrin to water for injection with 40% of the prescription amount, stir to completely dissolve the hydroxypropyl-β-cyclodextrin; accurately weigh 50g of voriconazole, dissolve it with 250mL of 2M hydrochloric acid, and control Warm at 50°C, add it into the dissolved hydroxypropyl-β-cyclodextrin aqueous solution, and stir while adding; continue to stir for 20 minutes to make voriconazole inclusion as complete as possible; accurately weigh 200g of mannitol, add the above-mentioned package Stir to dissolve the mixed soluti...
Embodiment 3
[0072] Embodiment 3 (specification 50mg)
[0073] The weight of each component in the prescription of preparing 1000 voriconazole freeze-dried powder injections for injection is as follows:
[0074] Voriconazole 50g Hydroxypropyl-β-cyclodextrin 600g
[0075] Mannitol 200g 2M hydrochloric acid 250ml
[0076] 2M NaOH to adjust the pH to 5.5 and water for injection to 5L
[0077] The preparation process is as follows:
[0078] Add 600g of hydroxypropyl-β-cyclodextrin to water for injection with 50% of the prescription amount, stir to completely dissolve the hydroxypropyl-β-cyclodextrin; accurately weigh 50g of voriconazole, dissolve it with 250mL of 2M hydrochloric acid, and control Warm at 40°C, add it to the dissolved hydroxypropyl-β-cyclodextrin aqueous solution, and stir while adding; continue to stir for 40 minutes to make voriconazole inclusion as complete as possible; accurately weigh 200g of mannitol, add the above-mentioned package Stir to dissolve the mixed solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com