Cadmium ion imprinted adsorbent, and preparation method and application thereof
A technology of imprinted adsorption and cadmium ions, which is applied in chemical instruments and methods, adsorption water/sewage treatment, and other chemical processes, can solve problems that have not been seen, achieve good stability, good reproducibility, and improve selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Preparation of cadmium ion imprinting adsorbent
[0018] (1) Add 1 mmol CdCl 2 , 2~4 mmol of methacrylic acid and 4-vinylpyridine functional monomers were dissolved in 15~20 mL of dimethyl sulfoxide solution, and the solution was magnetically stirred at room temperature for 1 h to form cadmium ion chelate;
[0019] (2) Dissolve 0.1~0.2 g of azobisisobutyronitrile and 20 mmol of ethylene glycol dimethacrylate in 2~3 mL of dimethyl sulfoxide solution. Under nitrogen protection, mix with the solution in step (1), and react in a water bath at 70°C for 24 h;
[0020] (3) The polymer formed in step (2) is first washed with ethanol, then washed with 2 M hydrochloric acid solution to remove template cadmium ions, and finally washed with deionized water twice until neutral, and dried in a drying oven for later use;
Embodiment 2
[0022] Effect of pH on Adsorption Performance of Cadmium Ion Imprinting Adsorbent
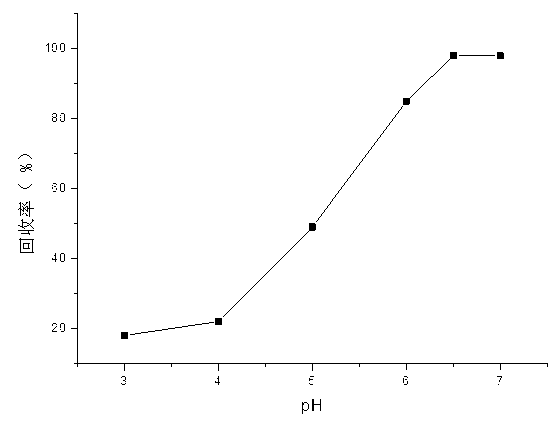
[0023] The absorption rate was measured by flame atomic absorption method (FAAS) through the static test of solutions with different pH values. figure 1 is the effect of pH on the adsorption performance of Cd(II) by cadmium ion imprinting adsorbent, the initial Cd(II) ion concentration: 1.0 mg / L; adsorbent dosage: 0.3g; shaking time: 30 min; temperature: 25°C; Sample volume: 100 mL.
Embodiment 3
[0025] Determination of maximum enrichment fold
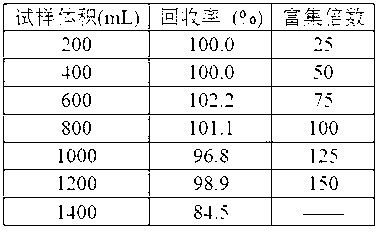
[0026] The enrichment performance of Cu(II) by cadmium ion-imprinted adsorbent under different sample volume conditions was investigated by dynamic experiment method. In the experiment, 200, 400, 600, 800, 1000, 1200 and 1400 mL sample solutions containing 10 μg Cd(II) were enriched by column extraction under the optimal extraction and elution conditions, and then the Cd(II) The determination of the recovery rate was used to study the maximum enrichment factor. The results are shown in Table 1. When the sample volume increased to 1400 mL, the recovery rate of Cd(II) dropped to 84.5%. This shows that the cadmium ion imprinting adsorbent has a good extraction and enrichment ability for Cd(II). Since 8 mL of hydrochloric acid solution can achieve quantitative recovery of Cd(II), the maximum enrichment factor is 150, which is expected to realize the extraction and enrichment of Cd(II) in the sample solution. Extraction and enrich...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com