Synthesis method of benzenetricarboxaldehyde compound
A technology of trimesicaldehyde and trimesicin is applied in the directions of oxidation to prepare carbonyl compounds, organic chemistry, etc., and achieves the effects of low synthesis cost, mild reaction conditions and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
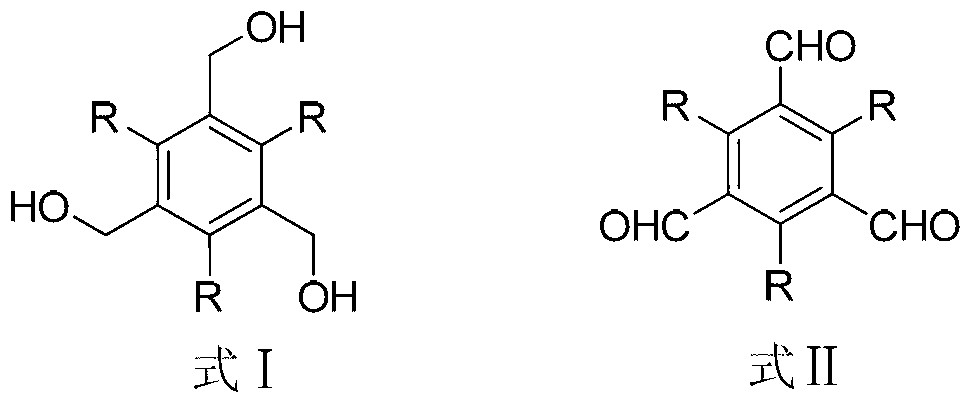
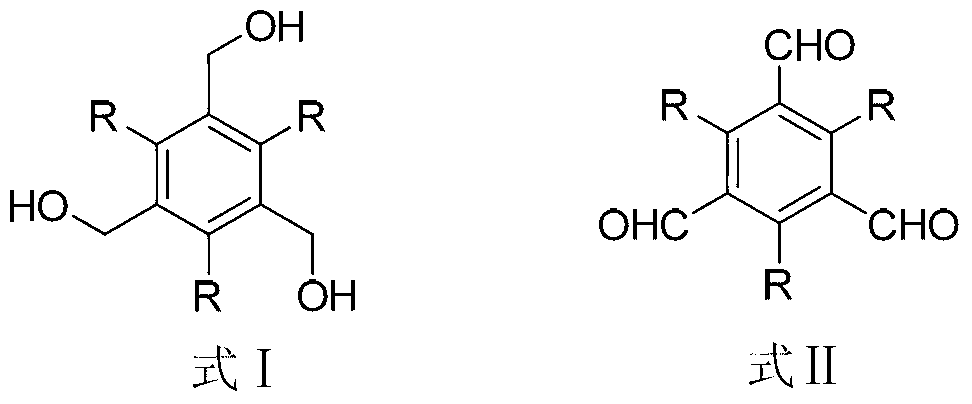
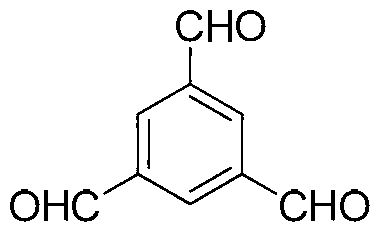
[0012] Taking the synthesis of 1,3,5-benzenetricarboxaldehyde as an example, its structural formula is as follows:
[0013]
[0014] Add 1.68g of 1,3,5-benzenetrimethanol and 0.32g of tetrabutylammonium bromide into a flask containing 100mL of dichloromethane, stir, and add 4.85g of potassium chromate into 150mL of aqueous sulfuric acid with a mass fraction of 30% , and then added dropwise to the flask, the molar ratio of 1,3,5-benzenetrimethanol to tetrabutylammonium bromide and potassium chromate was 1:0.1:2.5, and the reaction was carried out at room temperature for 2 hours. Wash with 10% sodium hydroxide aqueous solution and distilled water, wash until neutral, dry with anhydrous sodium sulfate, concentrate the solvent and separate by column chromatography to obtain white solid 1,3,5-benzenetricarbaldehyde, which is The yield is 97%, the melting point is 154-159°C, and the spectral data of the product are as follows:
[0015] 1 H NMR (300MHz, CDCl 3 ):δ=8.61(s,3H),10...
Embodiment 2
[0020] Taking the synthesis of 2,4,6-tribromo-1,3,5-benzenetricarbaldehyde as an example, its structural formula is as follows:
[0021]
[0022] In Example 1, the 1,3,5-benzenetrimethanol used was replaced with equimolar 2,4,6-tribromo-1,3,5-benzenetrimethanol, and other steps were the same as those in Example 1 to obtain White solid 2,4,6-tribromo-1,3,5-benzenetricarboxaldehyde, the yield is 89%, the melting point is >250°C, and the spectral data of the product are as follows:
[0023] 1 H NMR (300MHz, CDCl 3 ): δ=10.08(s, 3H).
[0024] 13 C NMR (75MHz, CDCl 3 ): δ=191.3, 134.2, 124.2.
[0025] IR(KBr)ν: 2887w, 1964w.
[0026] MS: theoretical value (C 9 H 3 Br 3 O 3 ) 395.7, the measured value is 395.7.
Embodiment 3
[0028] Taking the synthesis of 2,4,6-triethyl-1,3,5-benzenetricarbaldehyde as an example, its structural formula is as follows:
[0029]
[0030] In Example 1, the 1,3,5-benzenetrimethanol used was replaced with equimolar 2,4,6-triethyl-1,3,5-benzenetrimethanol, and other steps were the same as in Example 1, A white solid 2,4,6-triethyl-1,3,5-benzenetricarbaldehyde was obtained with a yield of 92%, and the spectral data of the product were as follows:
[0031] 1 H NMR (300MHz, CDCl 3 ): δ=10.58(s, 3H), 2.93(q, 6H), 1.22(t, 9H).
[0032] 13 C NMR (75MHz, CDCl 3 ): δ=194.5, 149.4, 134.6, 22.7, 16.7.
[0033] IR(KBr)ν: 2976w, 1696w, 1553w.
[0034] MS: Theoretical value C 15 H 18 O 3 (M+Na) + 269.1, the measured value is 269.1.
[0035] The 2,4,6-triethyl-1,3,5-benzenetricarbinol in this example was synthesized according to the literature method, and the specific synthesis method was as follows: at 0 °C, 1.5g of 1,3,5-triethylbenzene was dissolved in In 35 mL of dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com