7,4',5'-trihydroxyflavanone derivative and its application in preparation of liver cancer treatment medicines
A trihydroxyflavone, a technology for treating liver cancer, applied in the field of medicine, can solve the problems of poor selectivity, strong toxic and side effects, easy to produce drug resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I-01
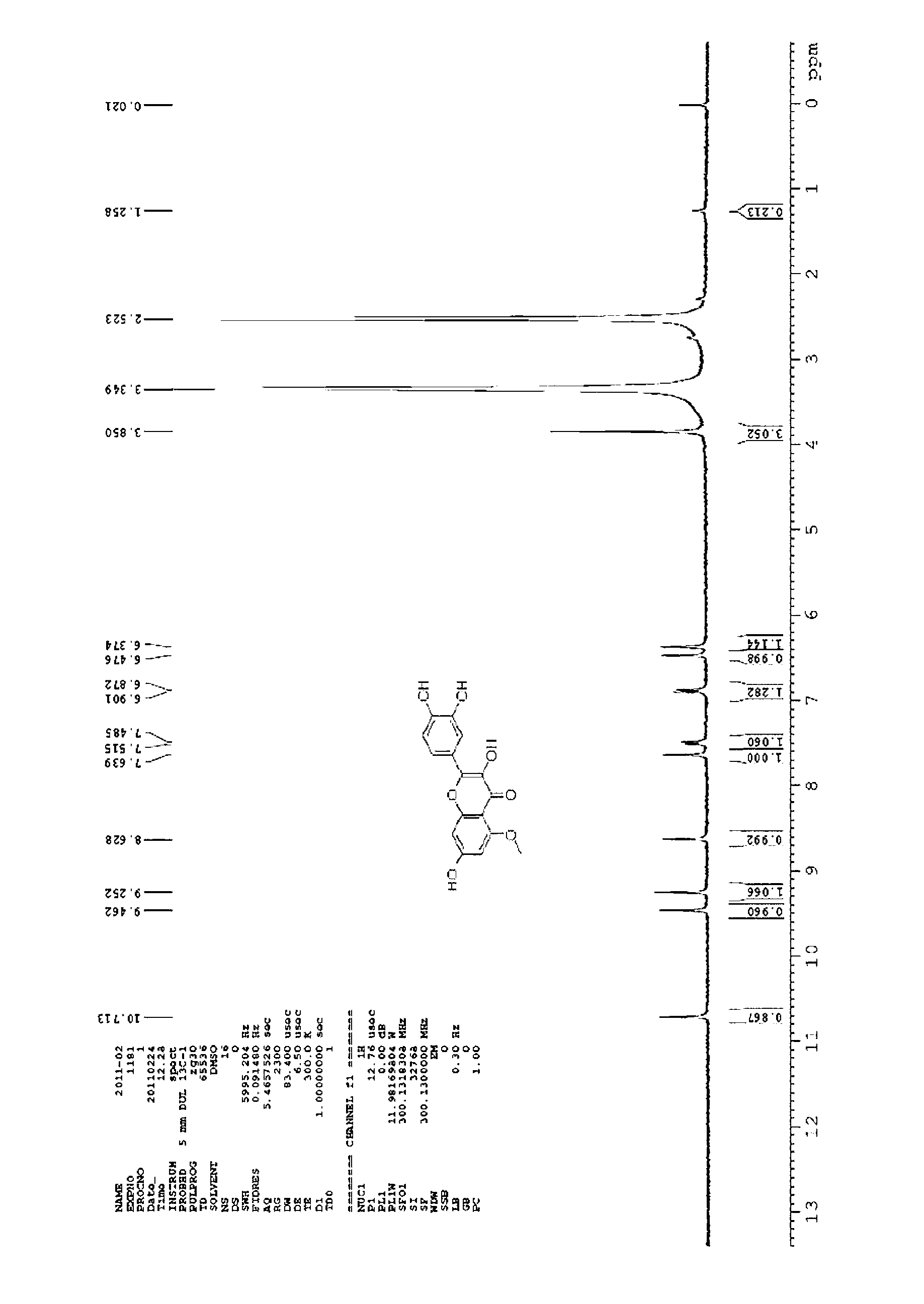
[0016] Example I-01: Synthesis of 2-(3,4-dihydroxy)-2,7-dihydroxy-5-methoxy-4H-benzopyran-4-one
[0017]
[0018] Under nitrogen protection conditions, 2-(3,4-dihydroxy)3,5,7-trihydroxy-4H-benzopyran-4-one (1g, 3.31mmol), benzyl bromide (1.98g, 11.58 mmol), potassium carbonate (1.60g, 11.58mmol) and 20mL of N,N-dimethylformamide were placed in a 100mL round bottom flask, and after stirring at room temperature for 12h, 100mL of water was added to the reaction system. Then it was extracted with ethyl acetate (100 mL×3), and the organic layers were combined. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and the organic phase was concentrated in vacuo. The residue was purified by silica gel column chromatography using chloroform as the eluent to obtain 450 mg of a yellow powdery solid with a yield of 21%.
[0019] 3,7-bis(benzyloxy)-2-[3,4-bisbenzyloxyphenyl]-5-hydroxy-4H-benzopyr-4-one (420mg, 0.63mmol) was treated with 5mL of N, N-...
Embodiment I-02
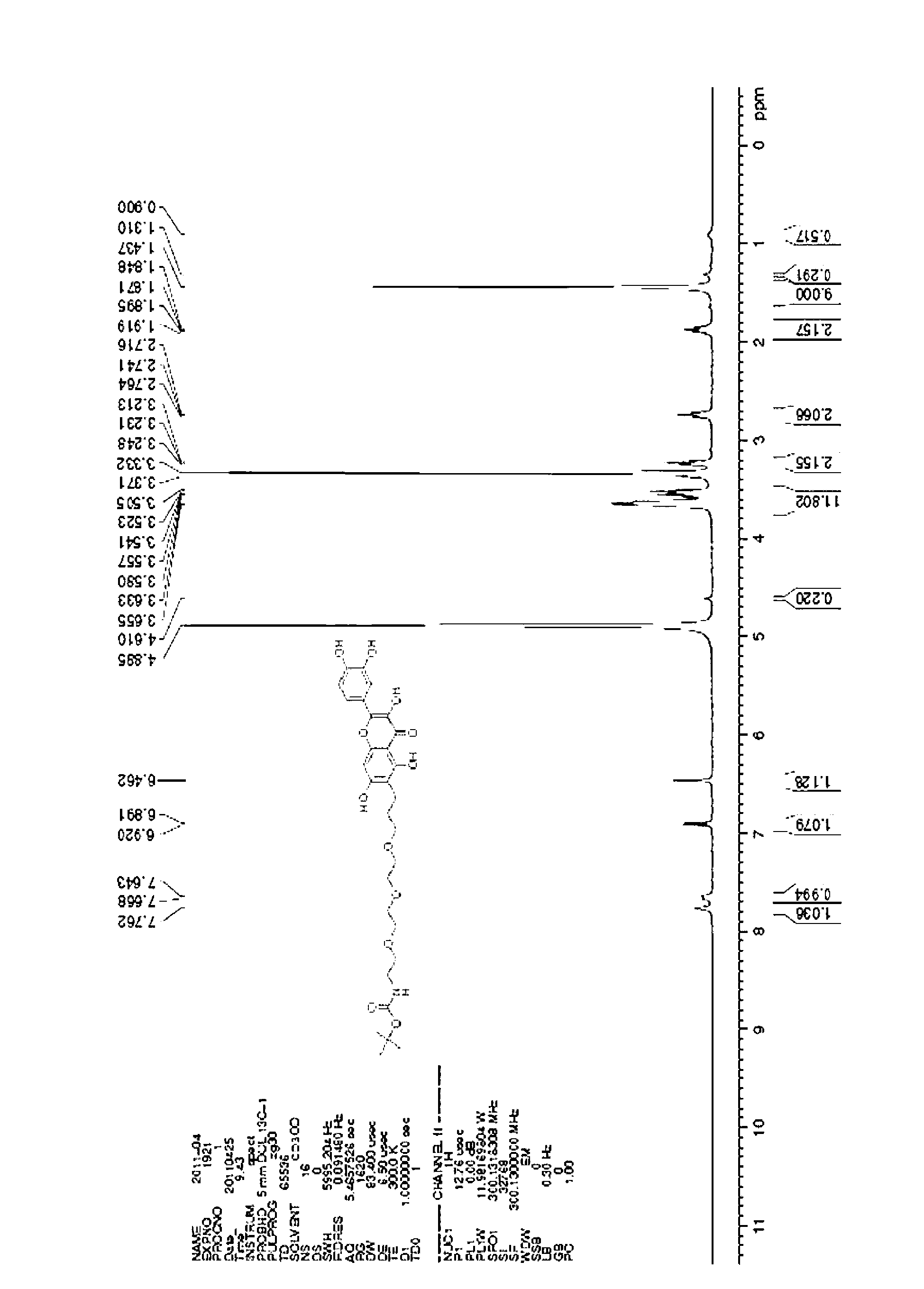
[0021] Example I-02: tert-butyl-N-[2-[2-(2-[3-[2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxyl-4-oxo Synthesis of -4H-benzopyran-6]propoxy]ethoxy)ethyl]carbamate
[0022]
[0023] Dissolve 2-(3,4-dihydroxy)3,5,7-trihydroxy-4H-chromen-4-one (1.0g, 3.31mmol) in 100mL of N,N-dimethylformamide In a 250mL round bottom flask, potassium carbonate (1.82g, 13.17mmol) was added simultaneously. Stir overnight at room temperature, and the reaction is complete. Add 200 mL of water to dilute the system, then extract with ethyl acetate (100 mL×2), combine the organic phases, and concentrate in vacuo. The residue was separated and purified by column chromatography, the developer was ethyl acetate / petroleum ether=1:20~1:10, and 1.2g of yellow solid 3,7-di(benzyloxy)-2-[3,4-di (Benzyloxy)phenyl]-5-hydroxy-4H-benzopyran-4-one, 55% yield.
[0024]3,7-bis(benzyloxy)-2-[3,4-bis(benzyloxy)phenyl]-5-hydroxyl-4H-benzopyran-4-one (7.0g, 10.56mmol) Dissolve in a 1000mL three-necked flask with a mixed ...
Embodiment I-03
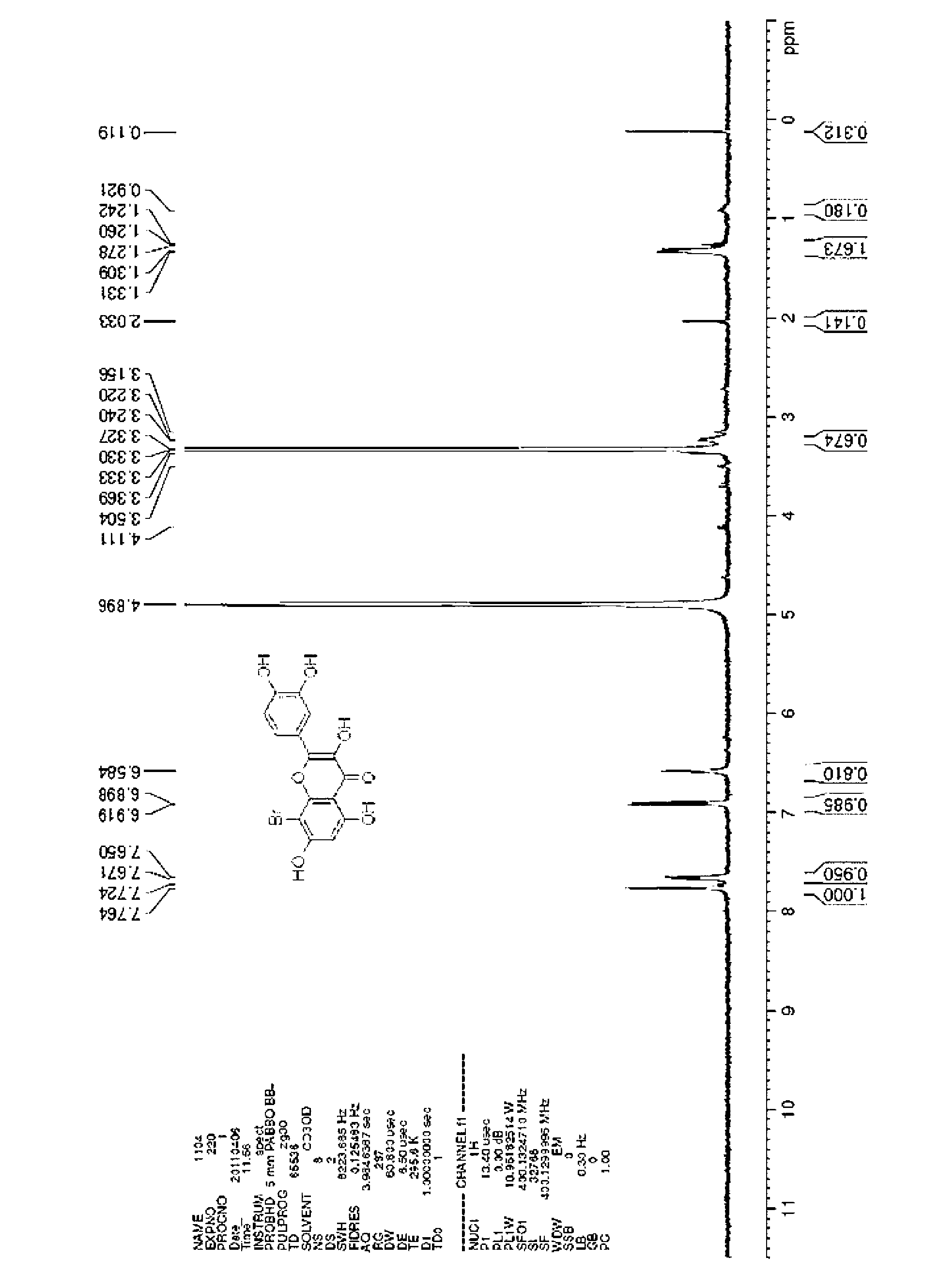
[0028] Example I-03: Synthesis of 8-bromo-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-benzopyran-4-one
[0029]
[0030] Dissolve 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one (500 mg, 1.65 mmol) in 10 mL of dichloromethane in a 50 mL round bottom In the flask, acetic anhydride (760mg, 7.44mmol) and pyridine (262mg, 3.31mmol) were added. Stir at room temperature for 3h, the reaction is complete. The reaction solution was directly concentrated in vacuo, and the resulting crude product was crystallized from a mixed solution of ethanol / water=10:1 to obtain 0.5 g of light yellow solid 3-(acetoxy)-2-[3,4-bis(acetoxy) Phenyl]-5-hydroxy-4-oxo-4H-chromene-7-acetate, yield 64%.
[0031] 3-(Acetoxy)-2-[3,4-bis(acetoxy)phenyl]-5-hydroxy-4-oxo-4H-chromene-7-acetate (50mg, 0.11 mmol) was dissolved in 5mL carbon tetrachloride in a 50mL round bottom flask, and N-bromosuccinimide (19mg, 0.11mmol) and (3mg, 0.01mmol) succinimide were added. The whole reaction system was stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com