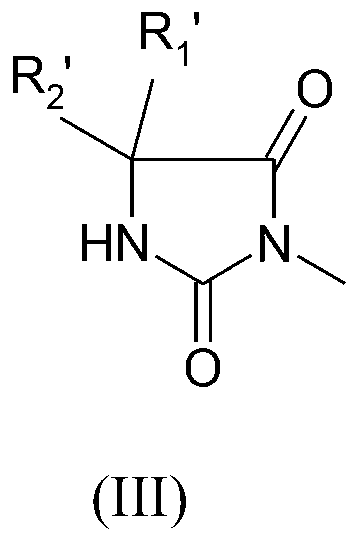
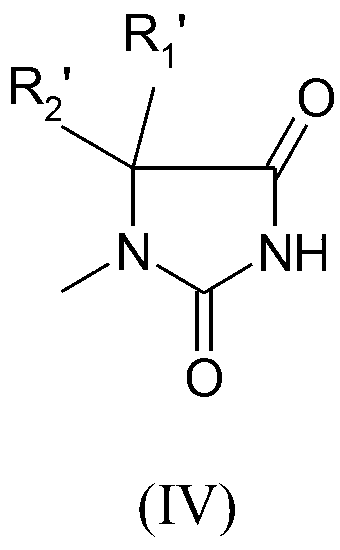
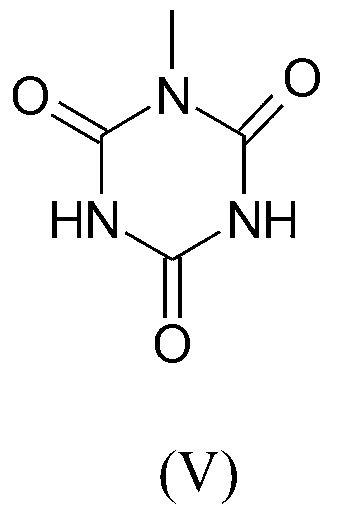
Preparation method of quaternized halamine antibacterial cotton fiber
A halide-amine antibacterial cotton and halide-amine technology are applied in fiber treatment, plant fiber, textiles and papermaking, etc., which can solve the problems of long reaction time and achieve the effects of cheap raw materials, easy availability of raw materials, and avoiding synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of N,N-Dimethylchloroethylamine Hydrochloride
[0033] 12.83g (0.106mol) of 99% thionyl chloride (SOCl 2) into a 250mL round-bottomed flask, and kept stirring, then weighed 8.91g (0.10mol) N,N-dimethylethanolamine, and added N,N-dimethylethanolamine dropwise under ice-bath conditions Add to the above-mentioned 250mL round bottom flask filled with thionyl chloride. After the dropwise addition, transfer the 250mL round bottom flask to a water bath at 35-50°C for 1 hour reaction, then add 100mL ethanol solution, and raise the temperature until the solution boils. After the reaction, the solution was cooled to crystallize, and then the solution was discarded, and the obtained crystal was vacuum-dried in a vacuum oven at 45° C. for 2 hours. Calculated by weighing, the productive rate is 83%.
Embodiment 2
[0035] Synthesis of 3-Dimethylaminoethyl-5,5-Dimethylhydantoin (DEADH)
[0036] Pour 6.4g (0.05mol) of 5,5-dimethylhydantoin and 8.64g (0.06mol) of N,N-dimethylchloroethylamine hydrochloride into a 500mL circle containing 100mL of 99.5% ethanol solution Add equimolar sodium ethoxide to the bottom flask respectively, then react the above two flasks under ice-bath conditions for 30min, then mix them, and stir them magnetically for 12h at 60°C. The reaction equation is shown below. After the reaction stopped, the by-product NaCl was removed by filtration, and the solvent ethanol was distilled off under reduced pressure. Then the obtained pale yellow solid was put into a vacuum oven and dried under vacuum for 4 h. According to the literature, TBPE is used as the indicator, TPB is used as the titrant, and the end point of the titration is when the titrated solution changes from red to yellow-green. Determination of its yield is 83.24%. The crude product of DEADH was separated a...
Embodiment 3
[0038] 3-Chloropropyltrimethoxysilane grafted on cotton fibers
[0039] Prepare an aqueous solution of 3-chloropropyltrimethoxysilane with a mass fraction of 6%, and stir at room temperature for 30 minutes to make 3-chloropropyltrimethoxysilane and deionized water completely miscible. Then soak 10 grams of cotton cloth fibers in the above solution for 15 minutes, and then place the cotton cloth in a 90° C. electric constant temperature blast drying oven to dry for 1 hour. Take it out and wash it with tap water and dry it for later use. The content of 3-chloropropyltrimethoxysilane bonded to the cotton fiber is determined by measuring the chlorine in organic chlorine, that is, the chlorine in C-Cl, by the oxygen bottle combustion method. Organochlorine content: 1.24%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com