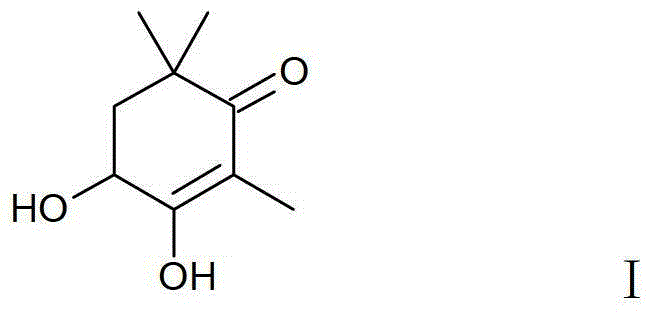
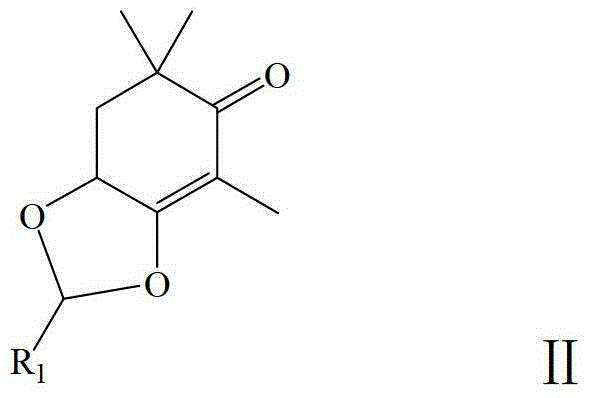
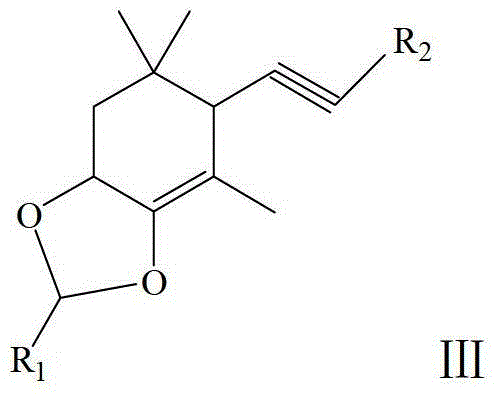
Method for Synthesizing Astaxanthin Intermediate Using 2,6,6-trimethyl-3,4-dihydroxy-2-cyclohexen-1-one
A dihydroxy, cyclohexene technology, applied in the preparation of heterocyclic compounds, organic chemistry and other directions, can solve the problems of difficult separation and purification, product isomerization, affecting product yield, etc., to improve reaction yield, good separation, The effect of reducing the concentration of acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A method for synthesizing an astaxanthin intermediate, the specific steps are as follows: add 170g (1.0mol) 2,6,6-trimethyl-3,4-dihydroxy-2-cyclohexen-1-one crystals to Put it into a 1000ml four-necked bottle equipped with a mechanical stirrer, a thermometer, a condenser tube and a drying tube. Add 350ml of n-heptane and stir to disperse, add 0.3g (1.6mmol) of p-toluenesulfonic acid, add 144g (2.0mol) of vinyl ethyl ether dropwise at room temperature, and drop it in 45 minutes. After stirring at room temperature for 30 minutes, raise the temperature to 60°C for 1 hour, add 1 g of triethylamine, and cool down to room temperature to obtain a reaction solution containing structural formula V, which can be directly reacted with alkynyllithium to obtain a product of structural formula VI ; Then add 200ml of 75% ethanol and 1g (0.01mol) of concentrated sulfuric acid in a 1000ml four-necked flask equipped with a mechanical stirrer, a thermometer, a condenser tube and a nitroge...
Embodiment 2
[0036] A method for synthesizing an astaxanthin intermediate, the specific steps are as follows: add 170g (1.0mol) crystal 2,6,6-trimethyl-3,4-dihydroxy-2-cyclohexen-1-one to Put it into a 1000ml four-necked bottle equipped with a mechanical stirrer, a thermometer, a condenser tube and a drying tube. Add 300ml cyclohexane and stir to disperse, add 0.6g (3.2mmol) p-toluenesulfonic acid, add 172g (2.0mol) propenyl ethyl ether dropwise at room temperature, and drop it in 45 minutes. After stirring at room temperature for 30 minutes, the temperature was raised to 70°C for 1 hour, 2 g of triethylamine was added, and the temperature was lowered to room temperature to obtain a reaction solution containing the structural formula VIII, which was directly reacted with alkynyl lithium to obtain the product of the structural formula IX ; Then add 200ml of 75% ethanol and 1g (0.01mol) of concentrated sulfuric acid in a 1000ml four-necked flask equipped with a mechanical stirrer, a thermome...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com