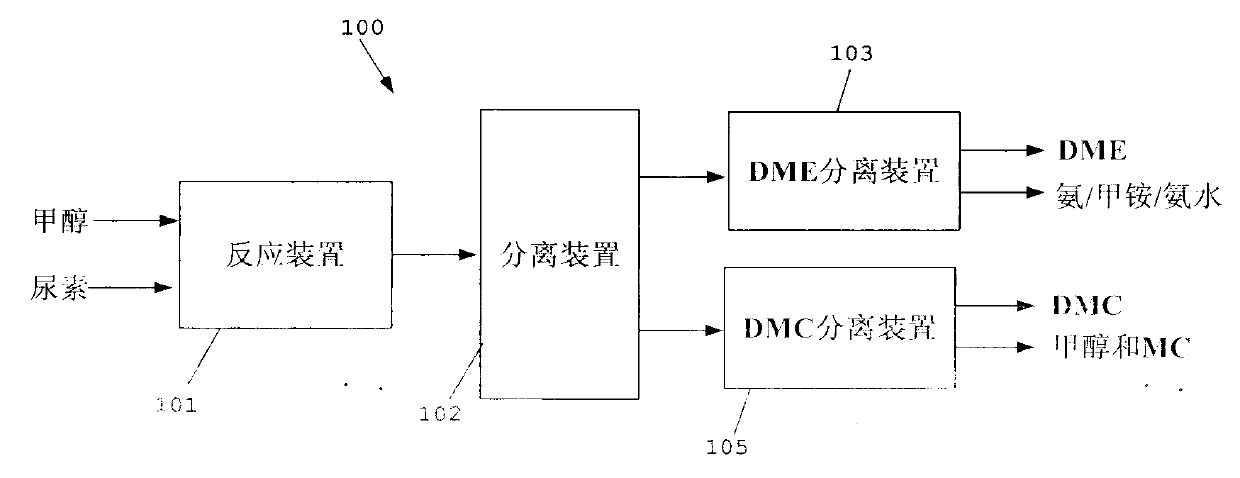
Method for separating dimethyl ether and recovering ammonia in co-production process for dimethyl carbonate and dimethyl ether via urea alcoholysis method
A technology for dimethyl carbonate and dimethyl ether, which is applied in the process of co-producing dimethyl carbonate and dimethyl ether by urea alcoholysis method to separate dimethyl ether and recover ammonia, can solve the problem of high total investment in production equipment and large-scale installations. Difficulty in transformation, impact on product economic benefits, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
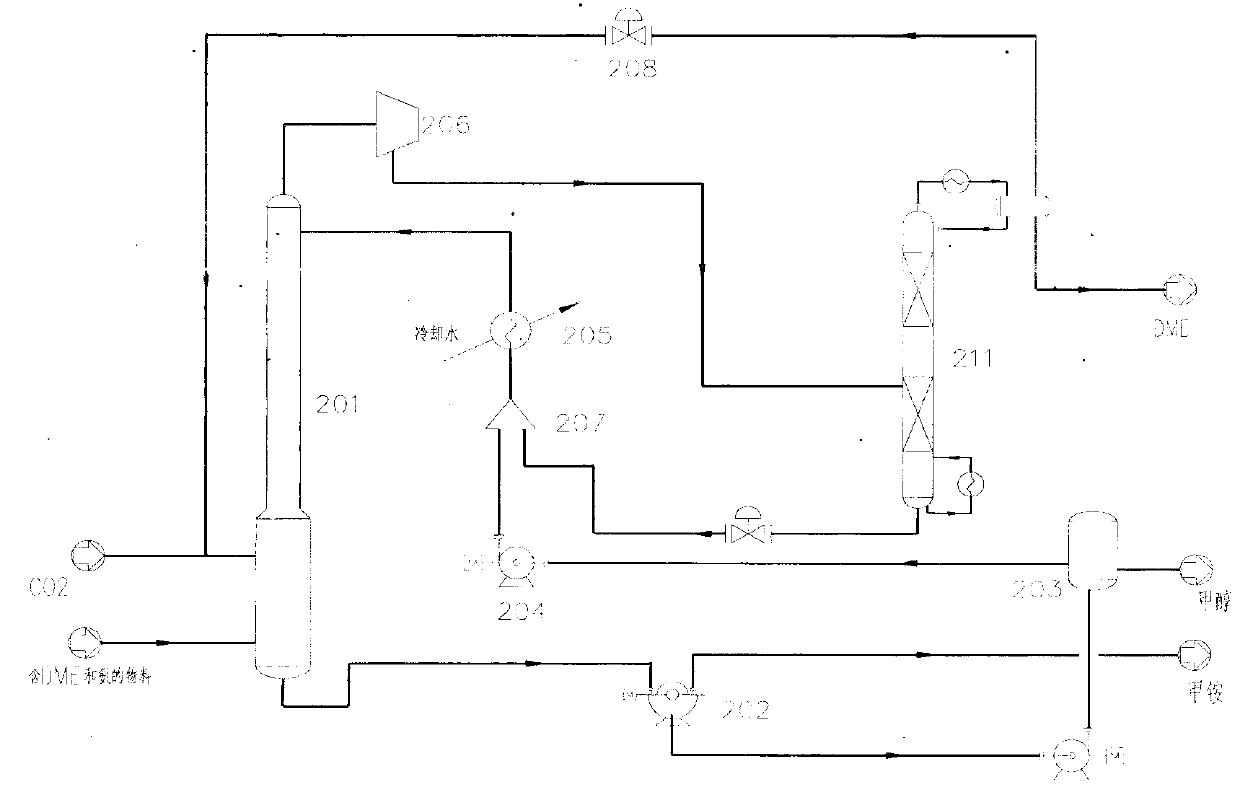
[0049] In Embodiment 1, the DME separation device 103a adopts the method of producing methylammonium by spraying methanol to separate DME and ammonia and recover ammonia. like figure 2 As shown, the DME separation unit 103 a includes a methanol washing column 201 and a DME-methanol rectifying column 211 .
[0050] The material containing DME and ammonia separated by the separation device 102 in the system 100 enters the methanol washing tower 201 . The DME-containing materials include: ammonia, DME, CO 2 , a small amount of methanol and DMC vapor and other gaseous substances. In one embodiment, the content of ammonia in the material is 39-55wt.%, the content of DME is 16-19wt.%, CO 2 The content of methanol is 17-18wt.%, the content of methanol is 7-22wt.%, and the content of DMC is 2-6wt.%.
[0051] Fresh CO supplemented from outside the system 2 Input methanol washing tower 201 after metering, to keep the CO in the methanol washing tower 201 2 excess.
[0052] Cold m...
Embodiment 2
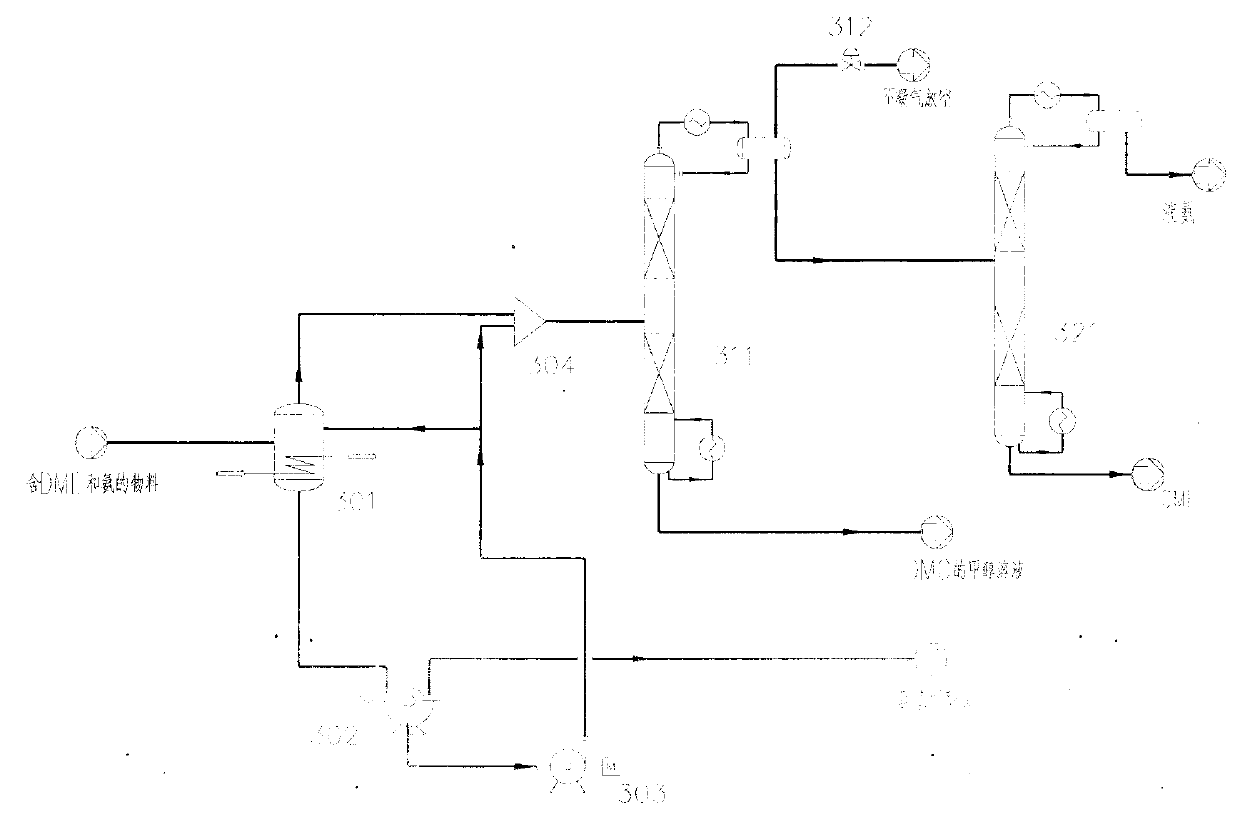
[0057] In the second embodiment, the DME separation device 103b adopts two rectification methods to separate methanol and recover liquid ammonia / DME. like image 3 As shown, the DME separation unit 103b includes a decarbonization tank 301 , a pre-rectification tower 311 and a main rectification tower 321 .
[0058] The material containing DME and ammonia separated by the separation device 102 in the system 100 enters the decarbonization tank 301 . The DME-containing material includes gaseous substances such as ammonia, DME, CO2, a small amount of methanol and DMC steam. In one embodiment, the content of ammonia in the material is 39-55wt.%, the content of DME is 16-19wt.%, the content of CO2 is 17-18wt.%, the content of methanol is 7-22wt.%, and the content of DMC is 2 ~6wt.%.
[0059] The decarbonization tank 301 has a built-in heat exchanger, which cools the incoming material to about 10°C. CO in the DME-containing material 2 And ammonia form ammonium carbamate crystals i...
Embodiment 3
[0063] In the third embodiment, the DME separation device 103c adopts the method of preparing ammonia water to separate DME and ammonia and recover ammonia. like Figure 4 As shown, the DME separation unit 103c includes a water absorption tower 401 and a flash tank 411 .
[0064] The material containing DME and ammonia separated by the separation device 102 in the system 100 enters the water absorption tower 401 . The DME-containing material includes gaseous substances such as ammonia, DME, CO2, a small amount of methanol and DMC steam. In one embodiment, the content of ammonia in the material is 39-55wt.%, the content of DME is 16-19wt.%, the content of CO2 is 17-18wt.%, the content of methanol is 7-22wt.%, and the content of DMC is 2 ~6wt.%.
[0065] The water absorption tower 401 uses fresh water from outside as absorbent. The material containing DME and ammonia flows upward from the bottom of the water absorption tower 401, and the fresh water from the outside is sent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com