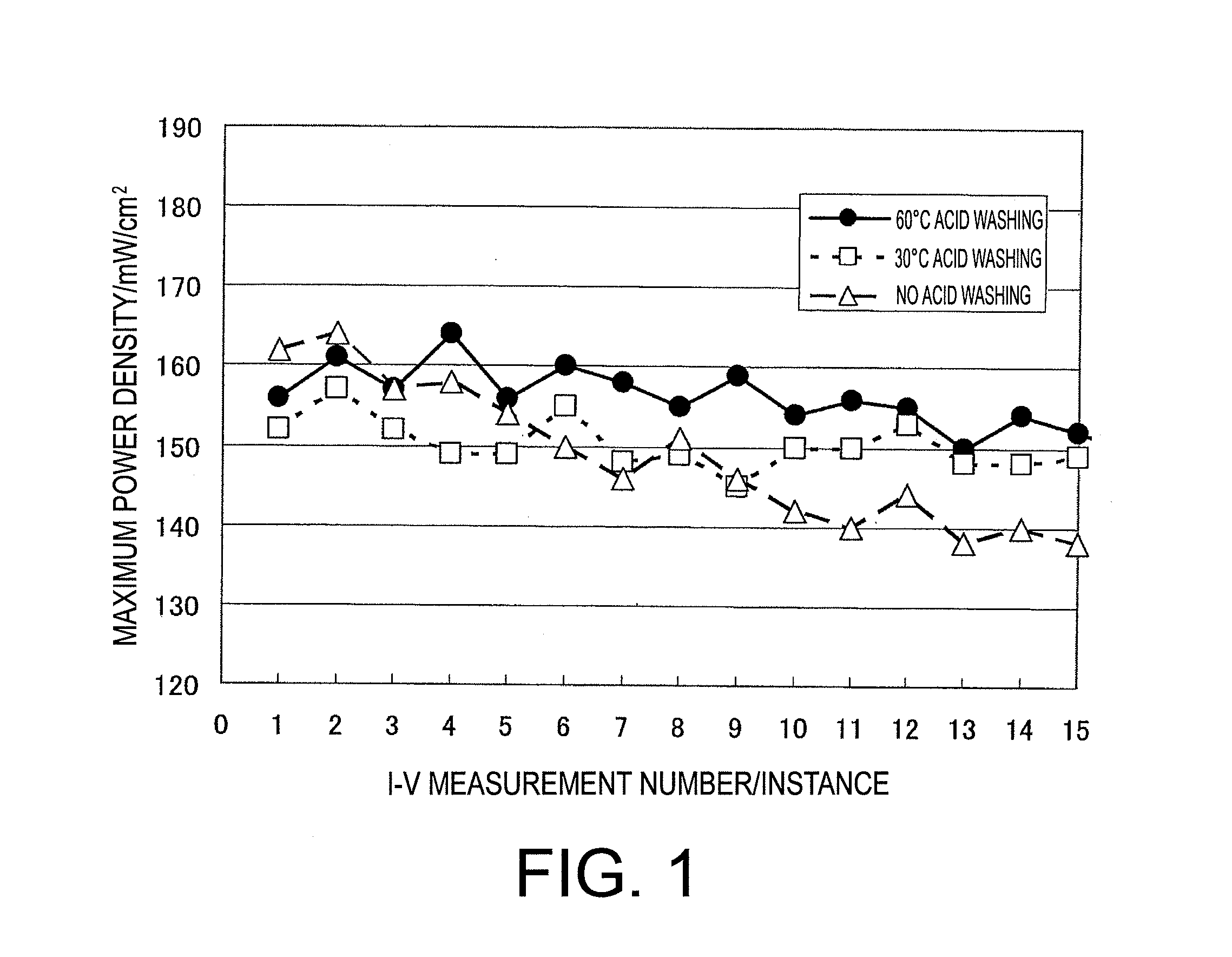
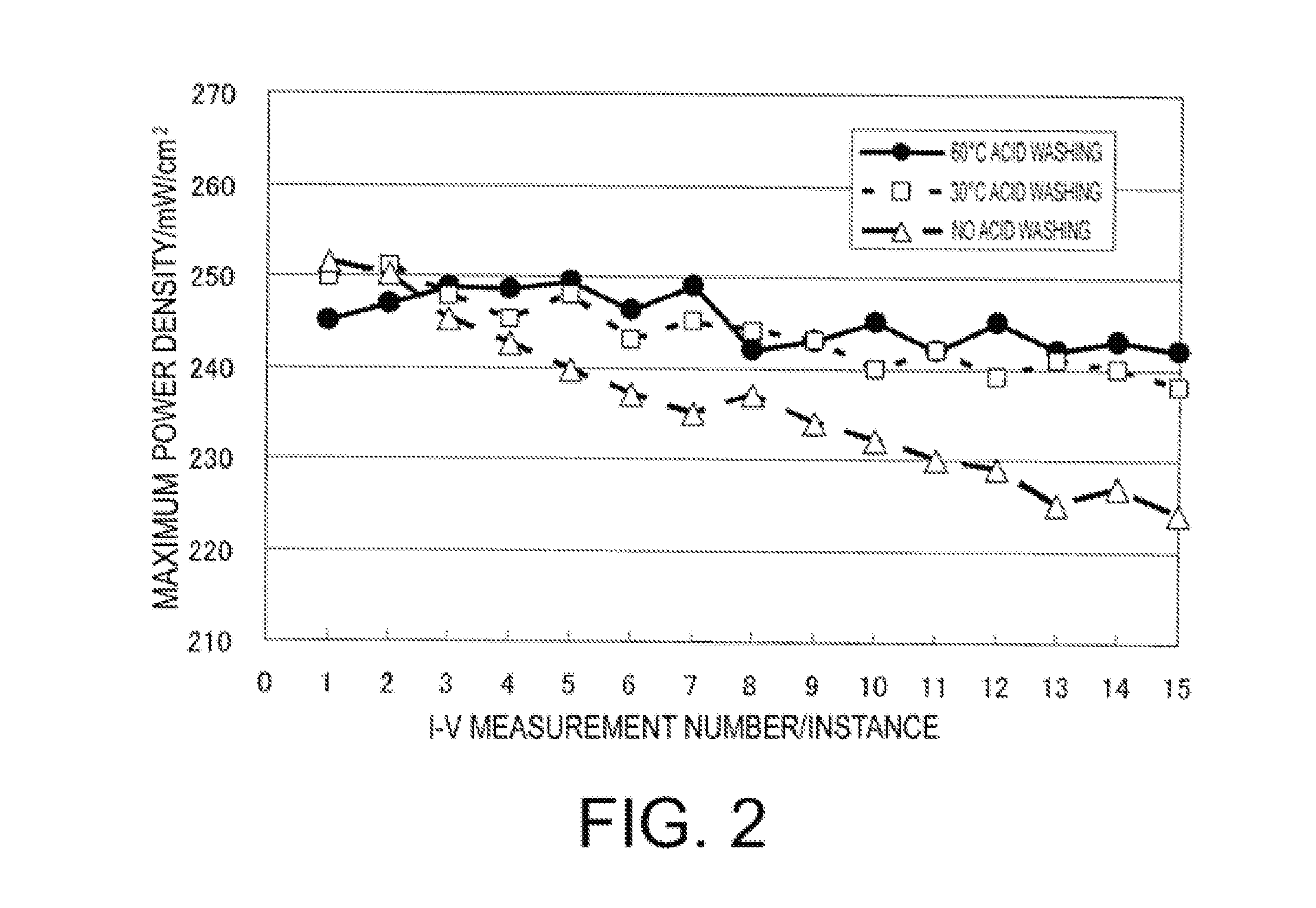
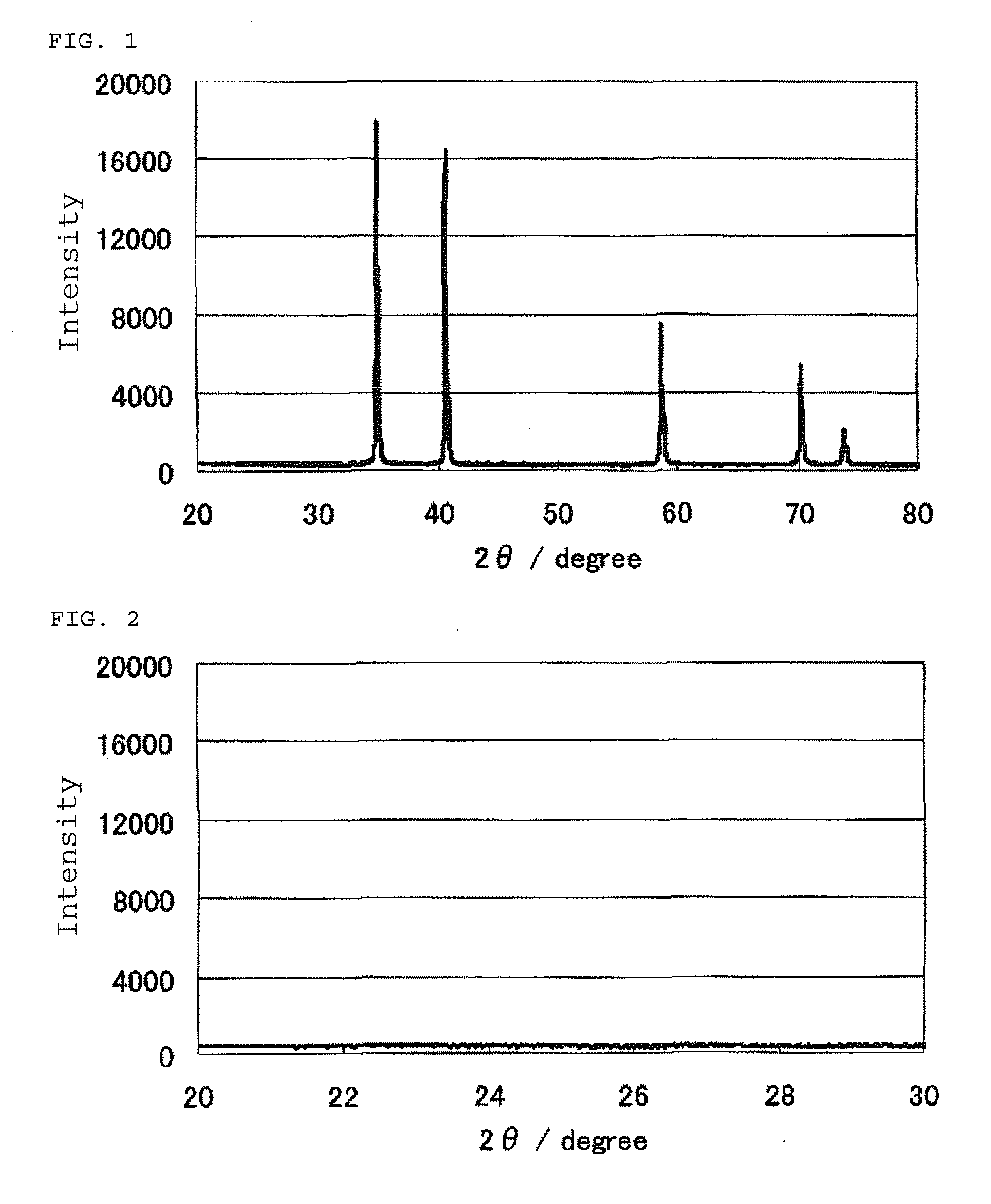
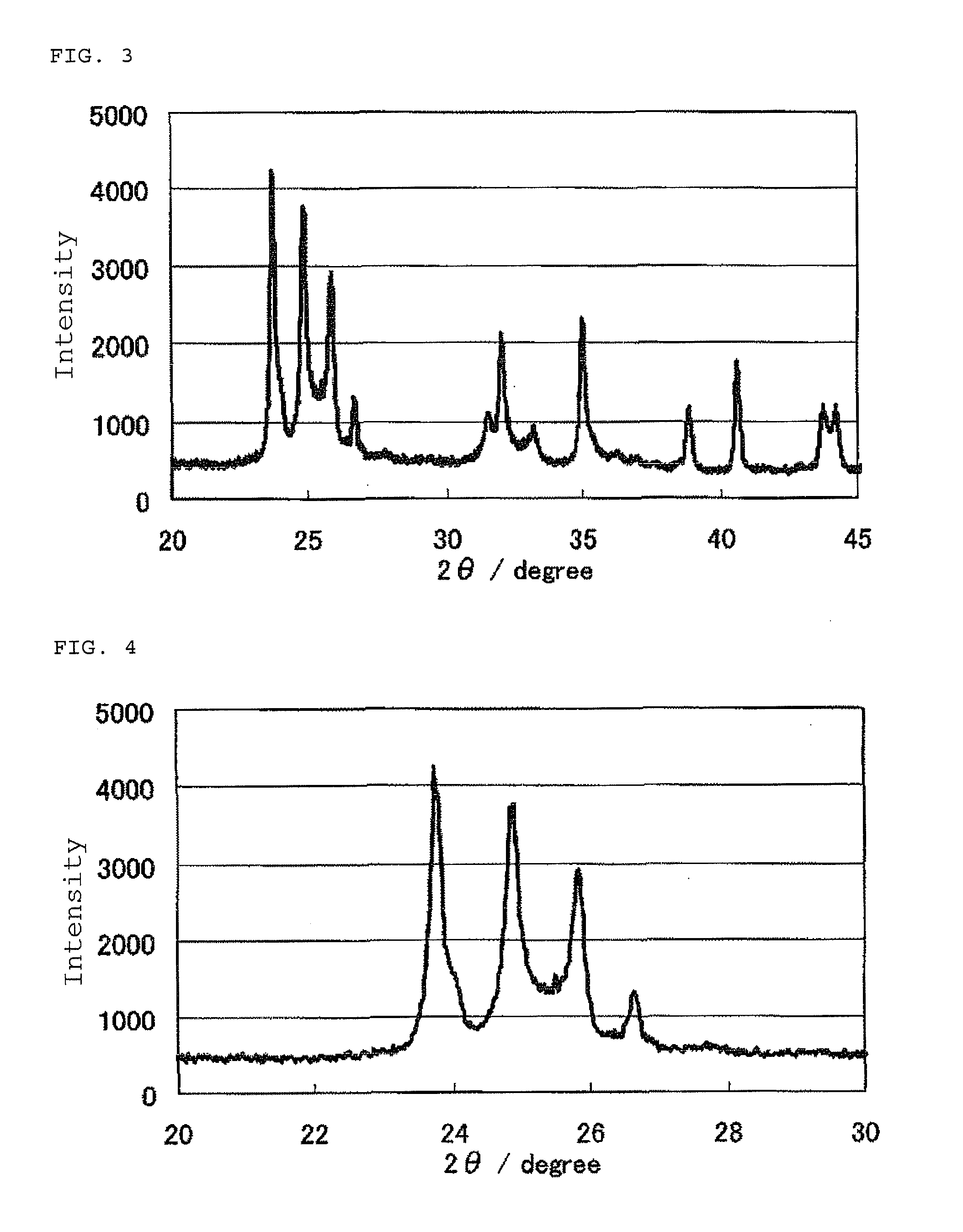
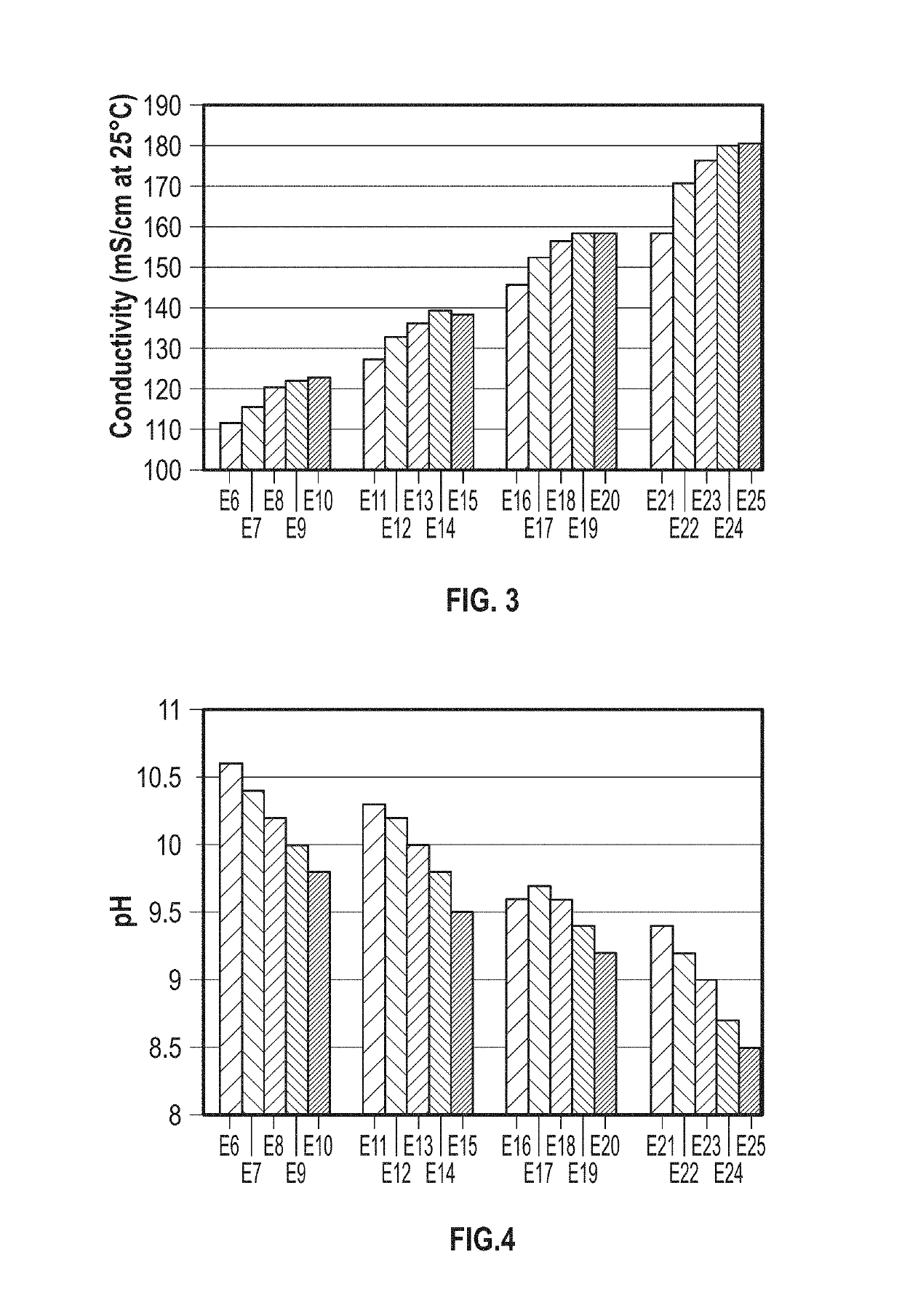
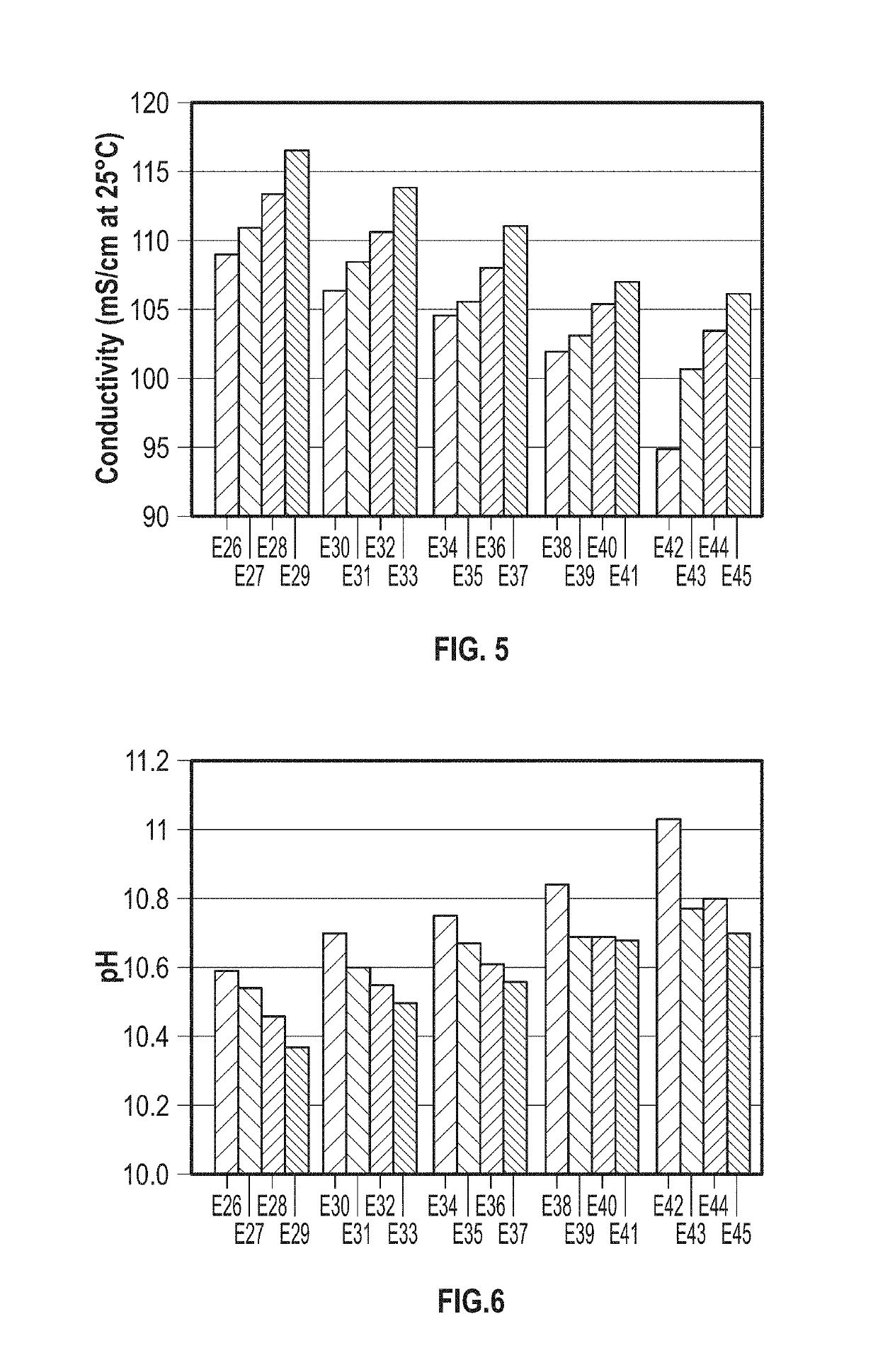
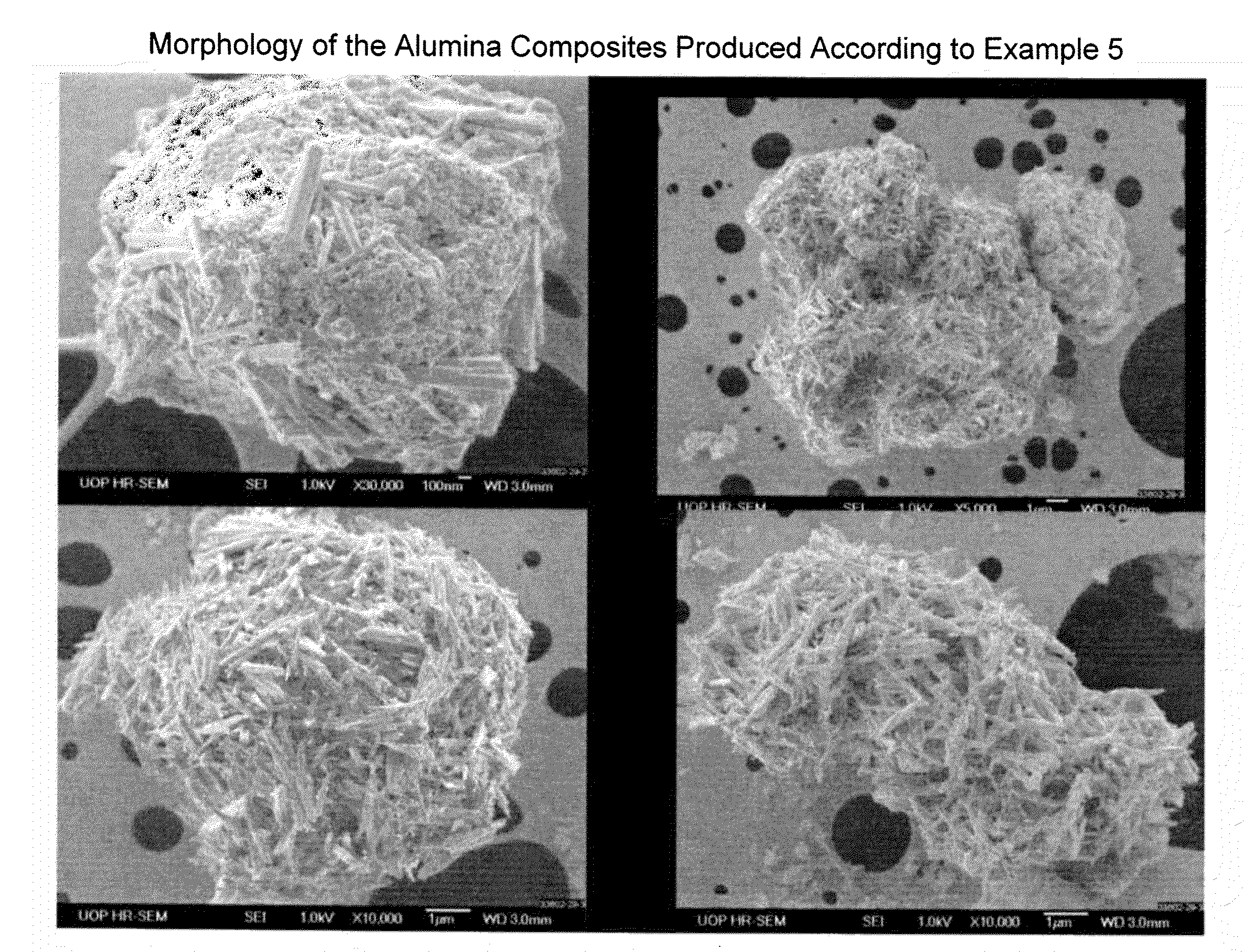Patents
Literature
61results about "Carbamic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
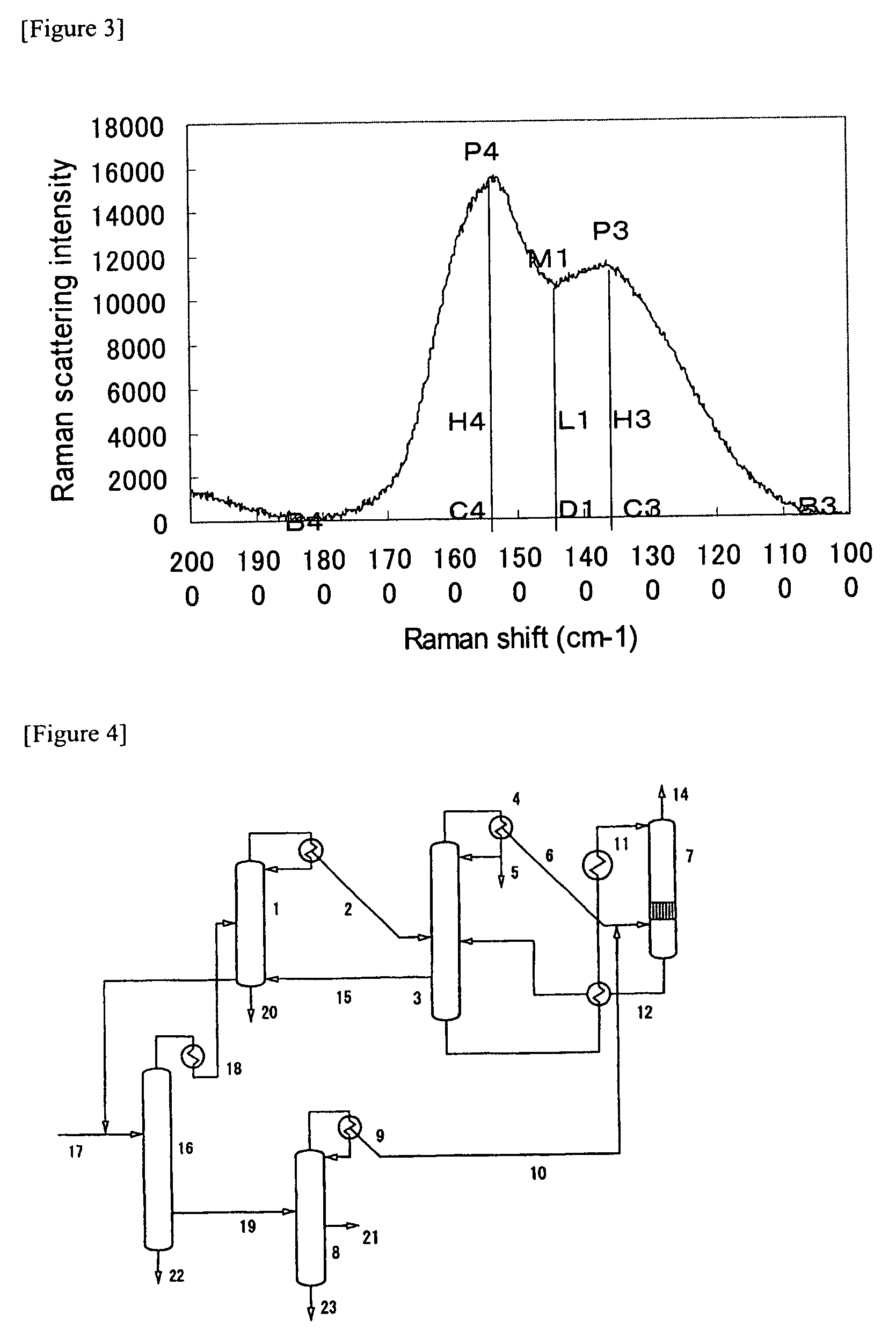
Synthesis of bis(fluorosulfonyl)imide
The present invention provides methods for producing bis(fluorosulfonyl) compounds of the formula:F—S(O)2—Z—S(O)2—F Iby contacting a nonfluorohalide compound of the formula:X—S(O)2—Z—S(O)2—Xwith bismuth trifluoride under conditions sufficient to produce the bis(fluorosulfonyl) compound of Formula I, where Z and X are those defined herein.
Owner:SES HLDG PTE LTD
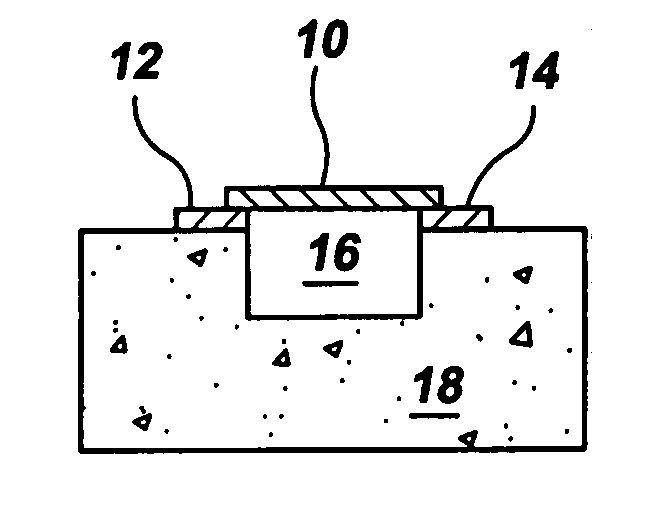
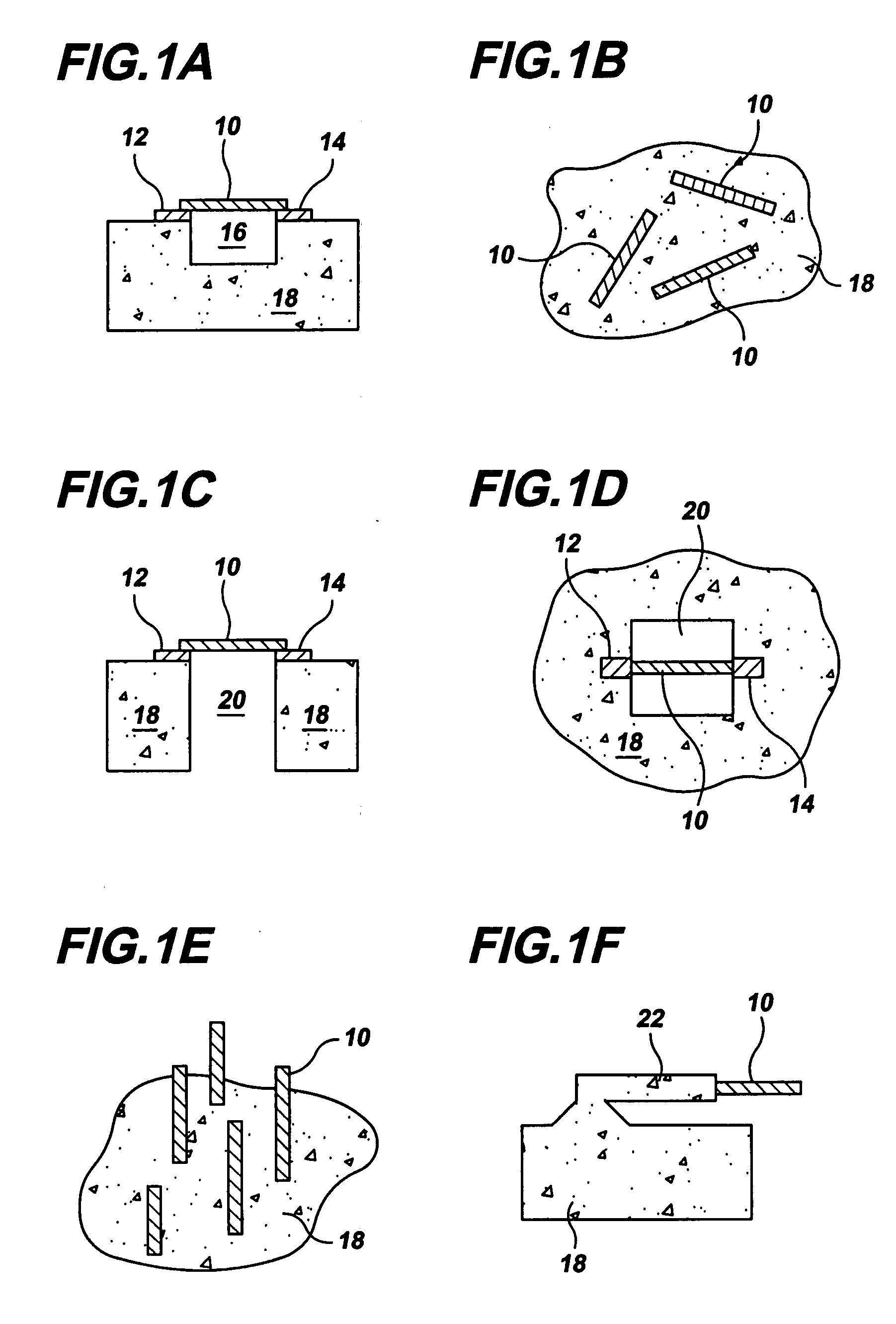
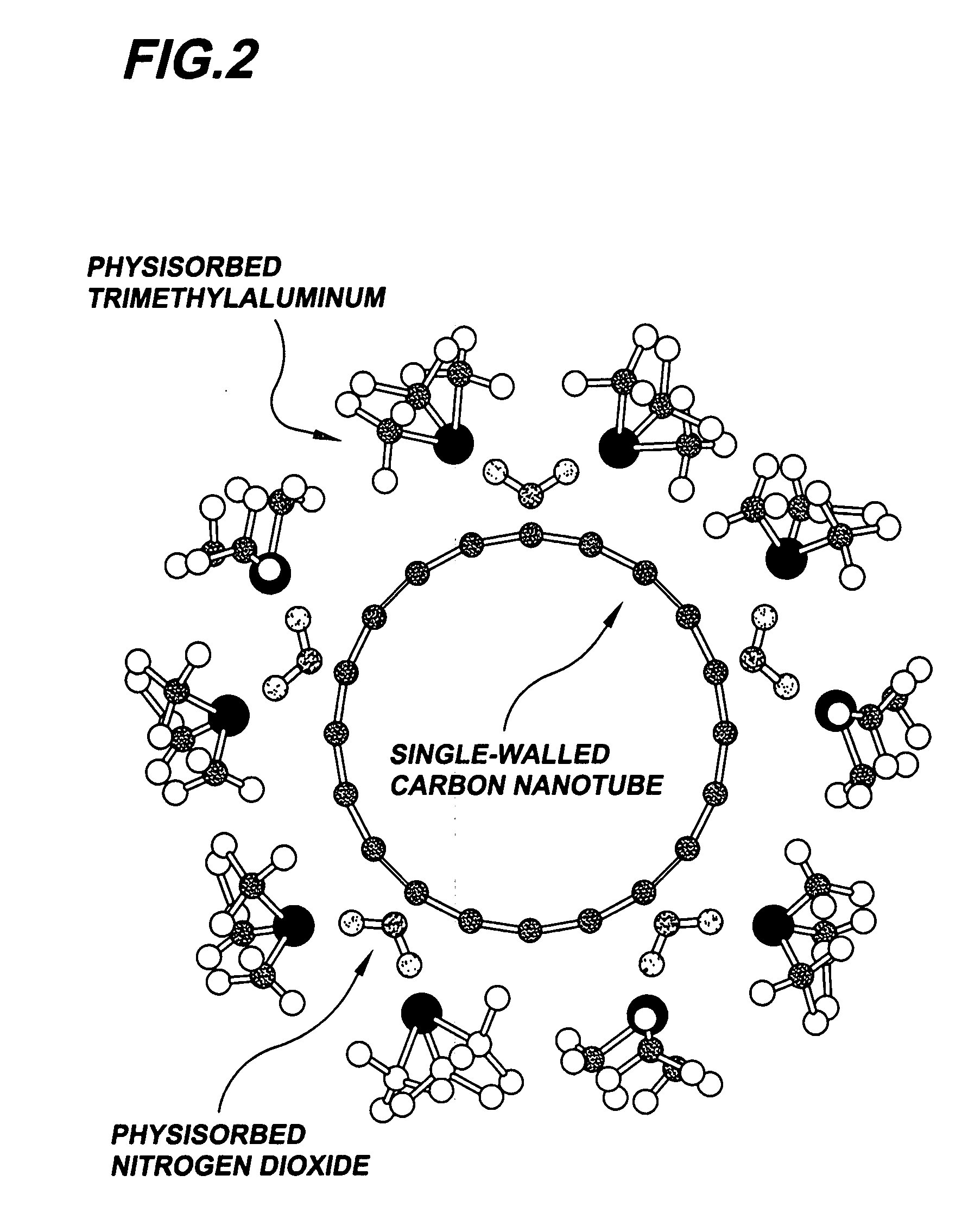
Gas-phase functionalization of carbon nanotubes
ActiveUS20080296537A1Remarkable and mechanical and optical propertyMaterial nanotechnologyAluminium compoundsGas phaseDesorption
In a method for functionalizing a carbon nanotube surface, the nanotube surface is exposed to at least one vapor including at least one functionalization species that non-covalently bonds to the nanotube surface, providing chemically functional groups at the nanotube surface, producing a functionalized nanotube surface. A functionalized nanotube surface can be exposed to at least one vapor stabilization species that reacts with the functionalization layer to form a stabilization layer that stabilizes the functionalization layer against desorption from the nanotube surface while providing chemically functional groups at the nanotube surface, producing a stabilized nanotube surface. The stabilized nanotube surface can be exposed to at least one material layer precursor species that deposits a material layer on the stabilized nanotube surface.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
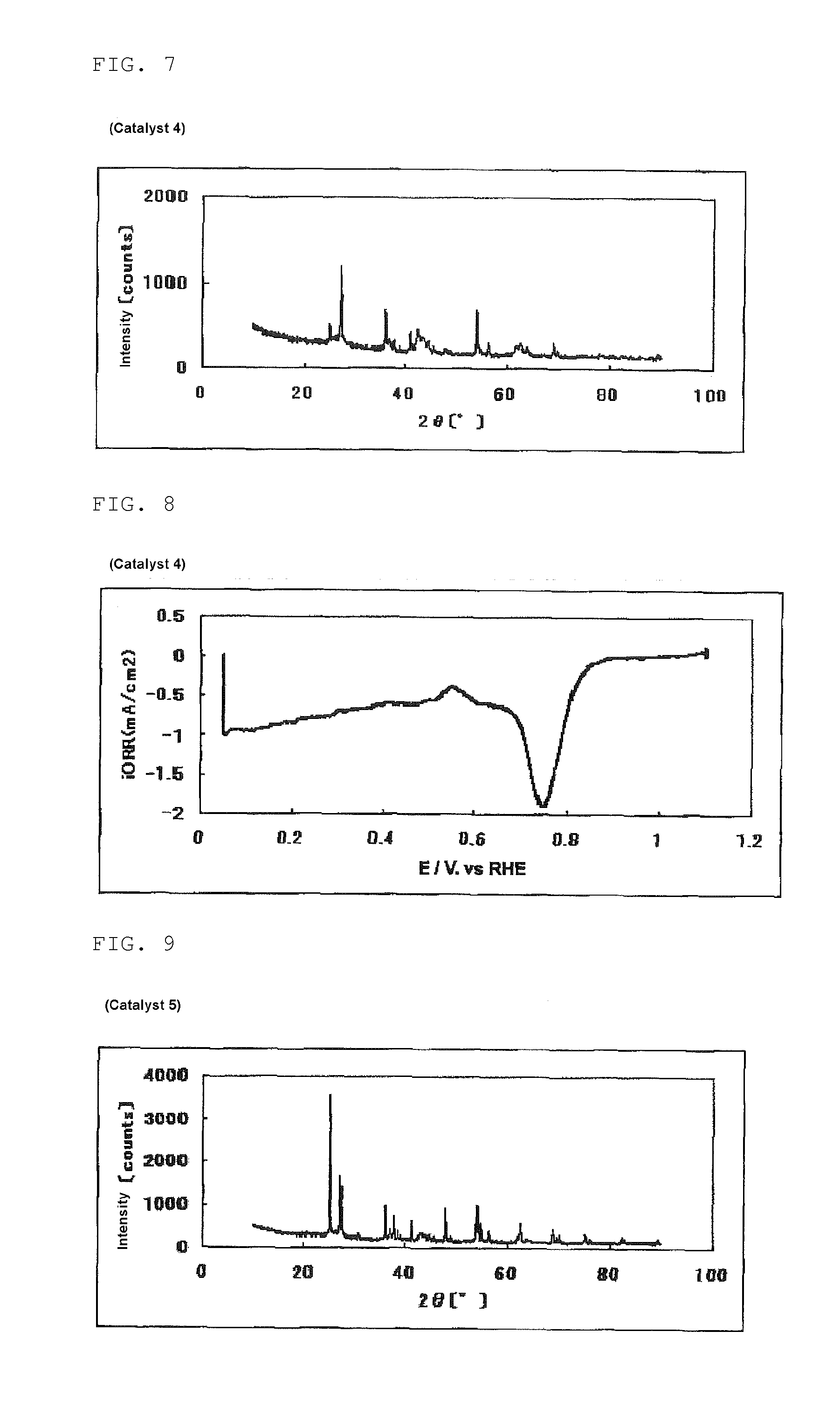
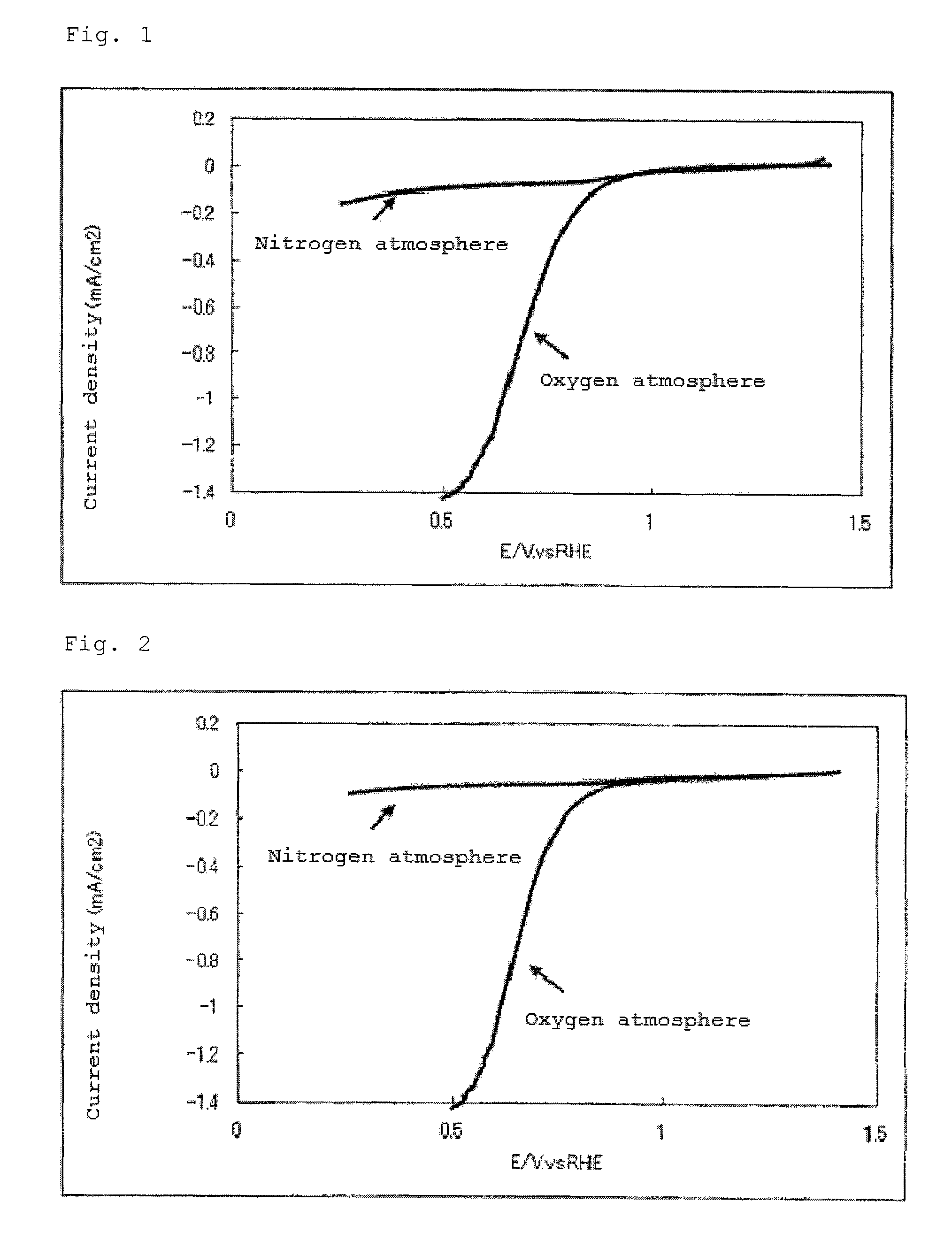
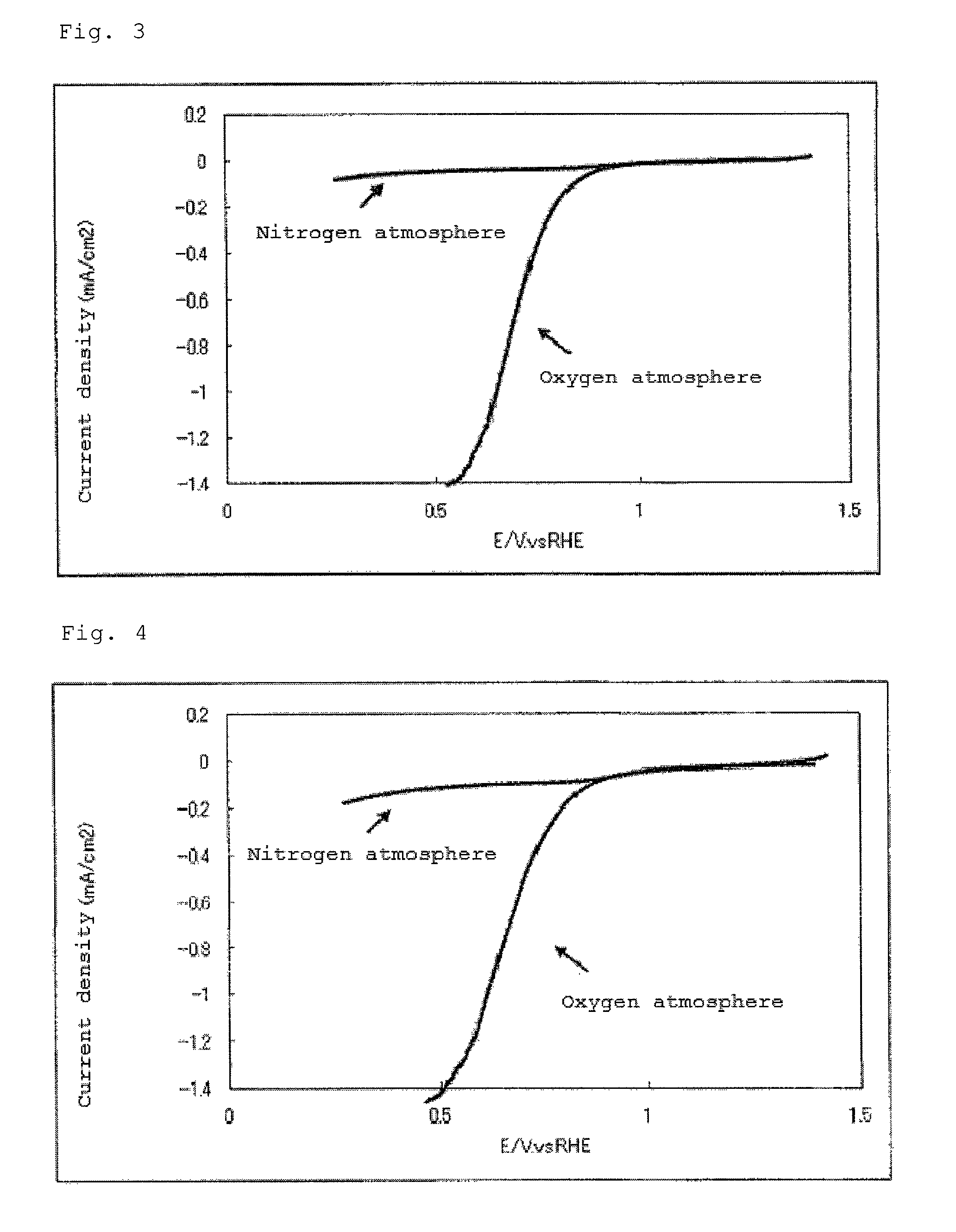
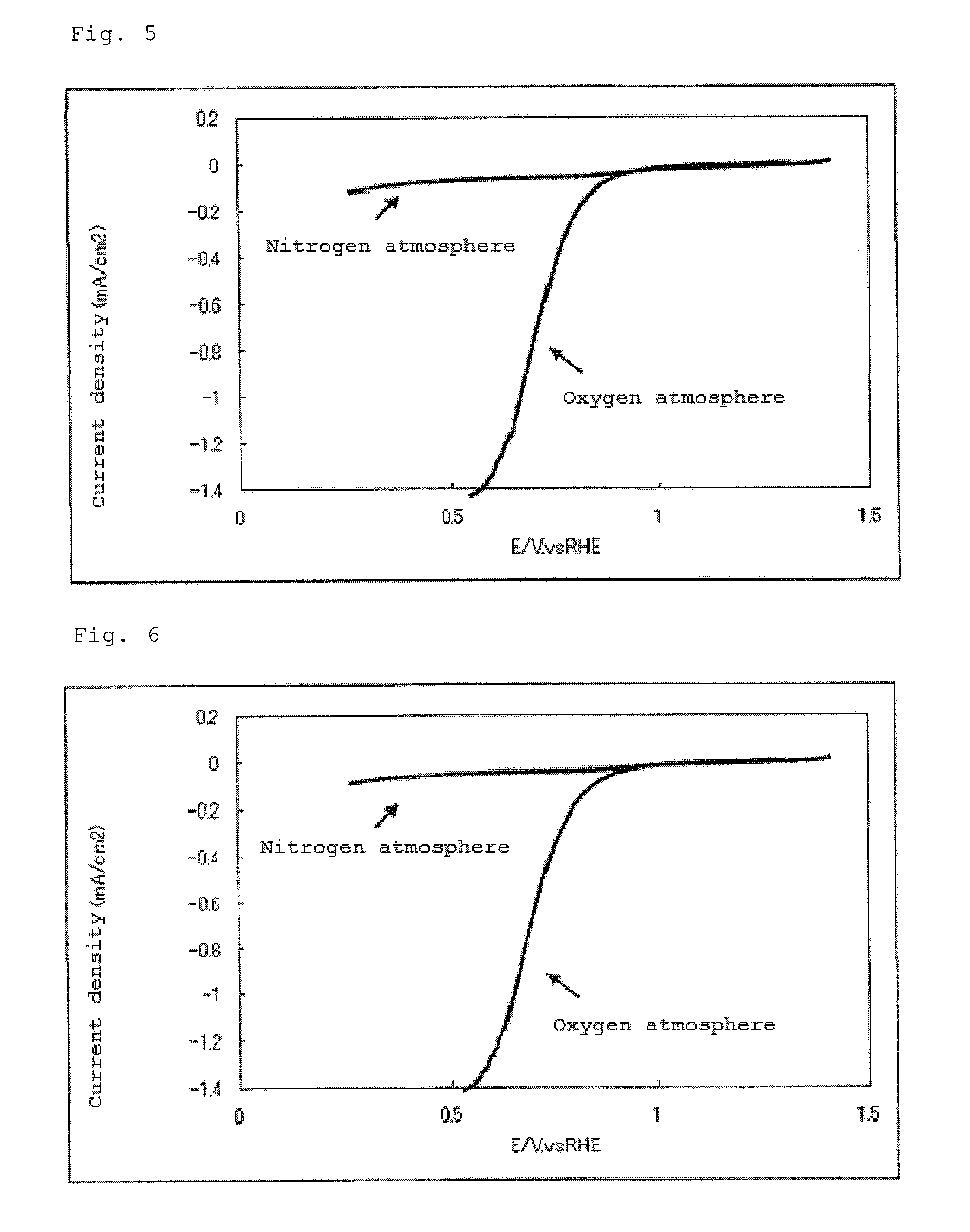
Catalyst carrier, catalyst and process for producing the same
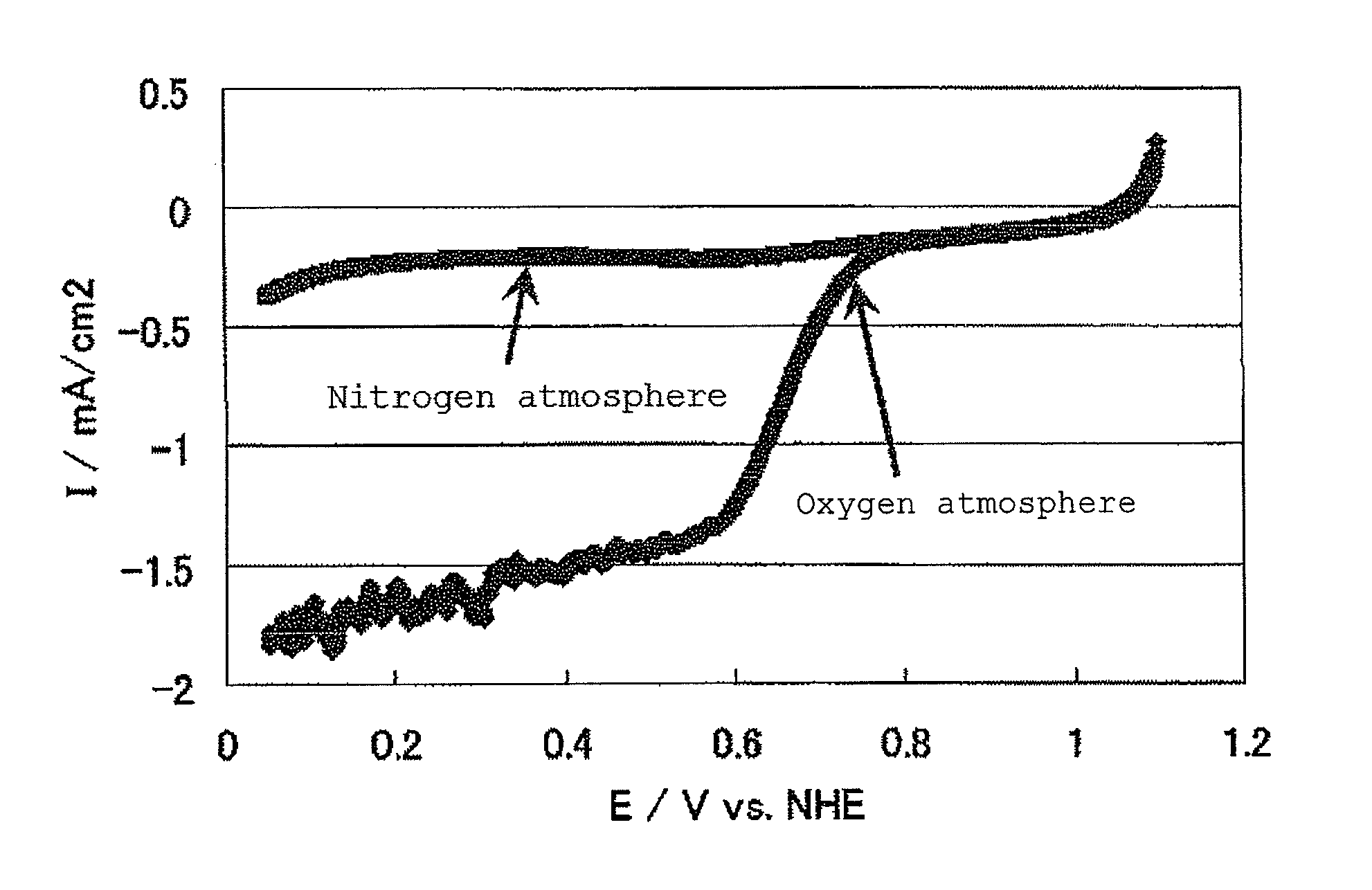
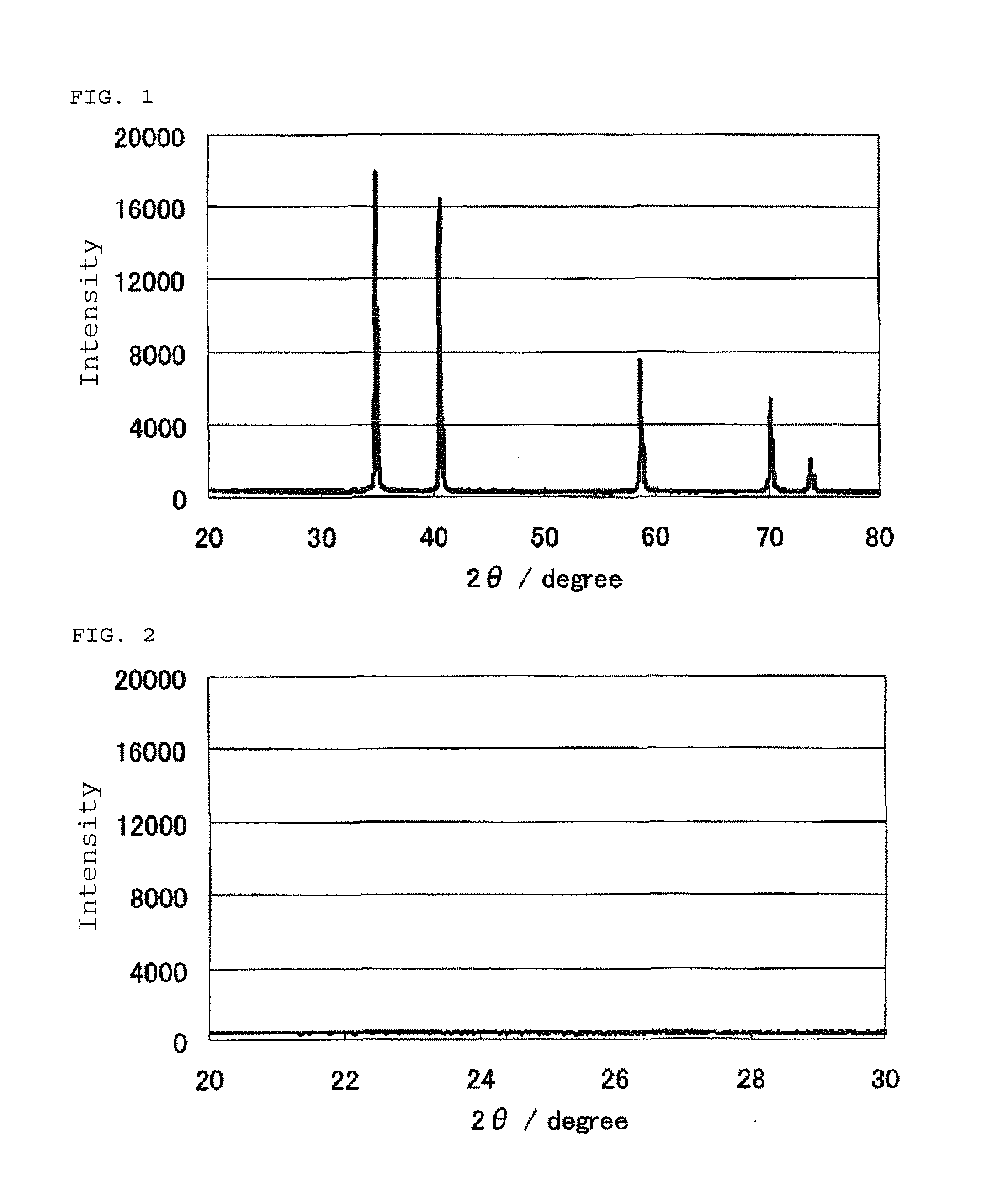
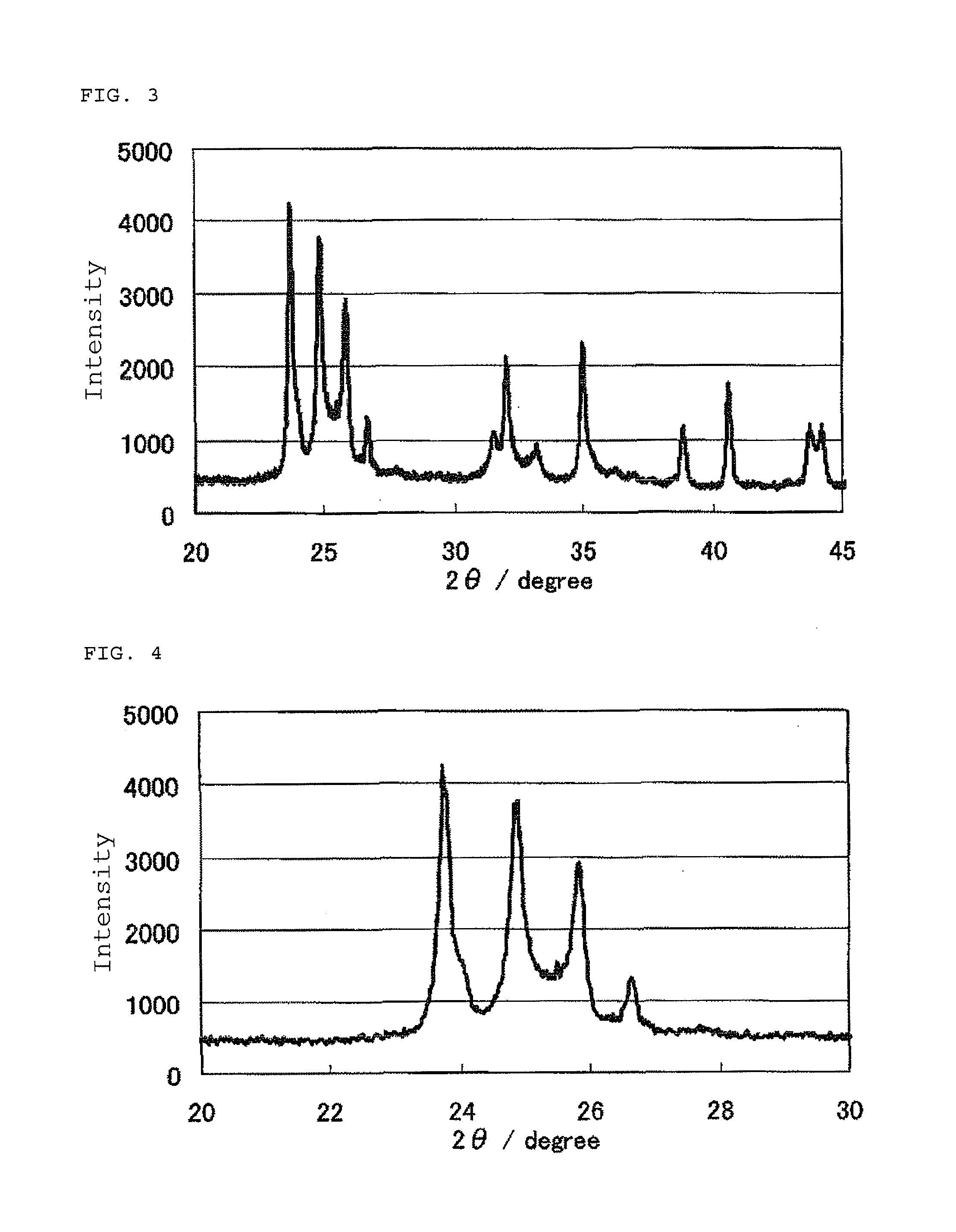
InactiveUS20100331172A1Improve heat resistanceHigh catalytic ability without increasing the specific surface areaCatalyst carriersCell electrodesIndiumCerium
The present invention provides a catalyst carrier having excellent durability and capable of attaining high catalytic ability without increasing the specific surface area thereof, and a catalyst obtainable by using the catalyst carrier. The catalyst carrier of the present invention comprises a metal oxycarbonitride, preferably the metal contained in the metal oxycarbonitride comprises at least one selected from the group consisting of niobium, tin, indium, platinum, tantalum, zirconium, copper, iron, tungsten, chromium, molybdenum, hafnium, titanium, vanadium, cobalt, manganese, cerium, mercury, plutonium, gold, silver, iridium, palladium, yttrium, ruthenium, lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, and nickel. Moreover, the catalyst of the present invention comprises the catalyst carrier and a catalyst metal supported on the catalyst carrier.
Owner:SHOWA DENKO KK
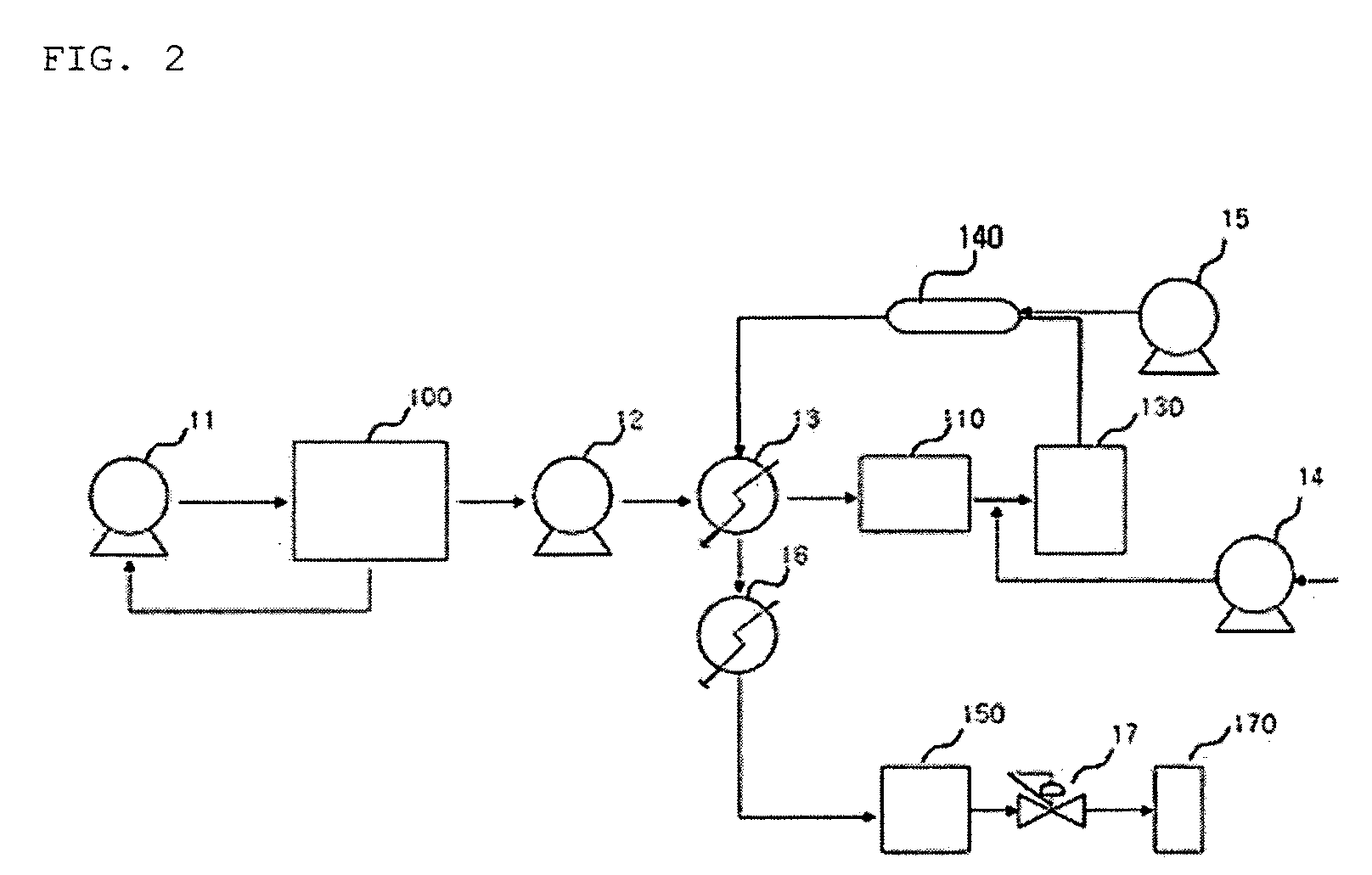
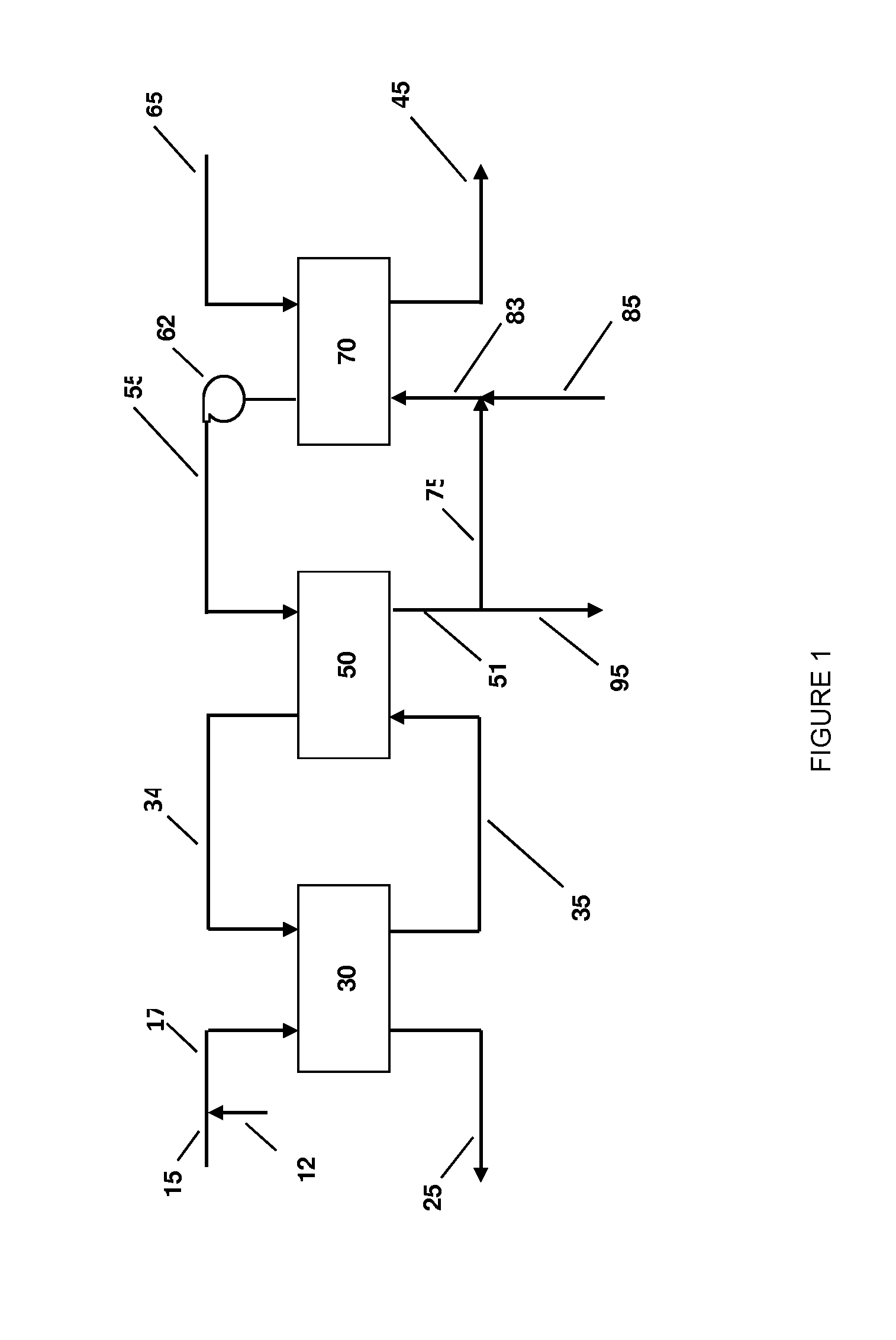
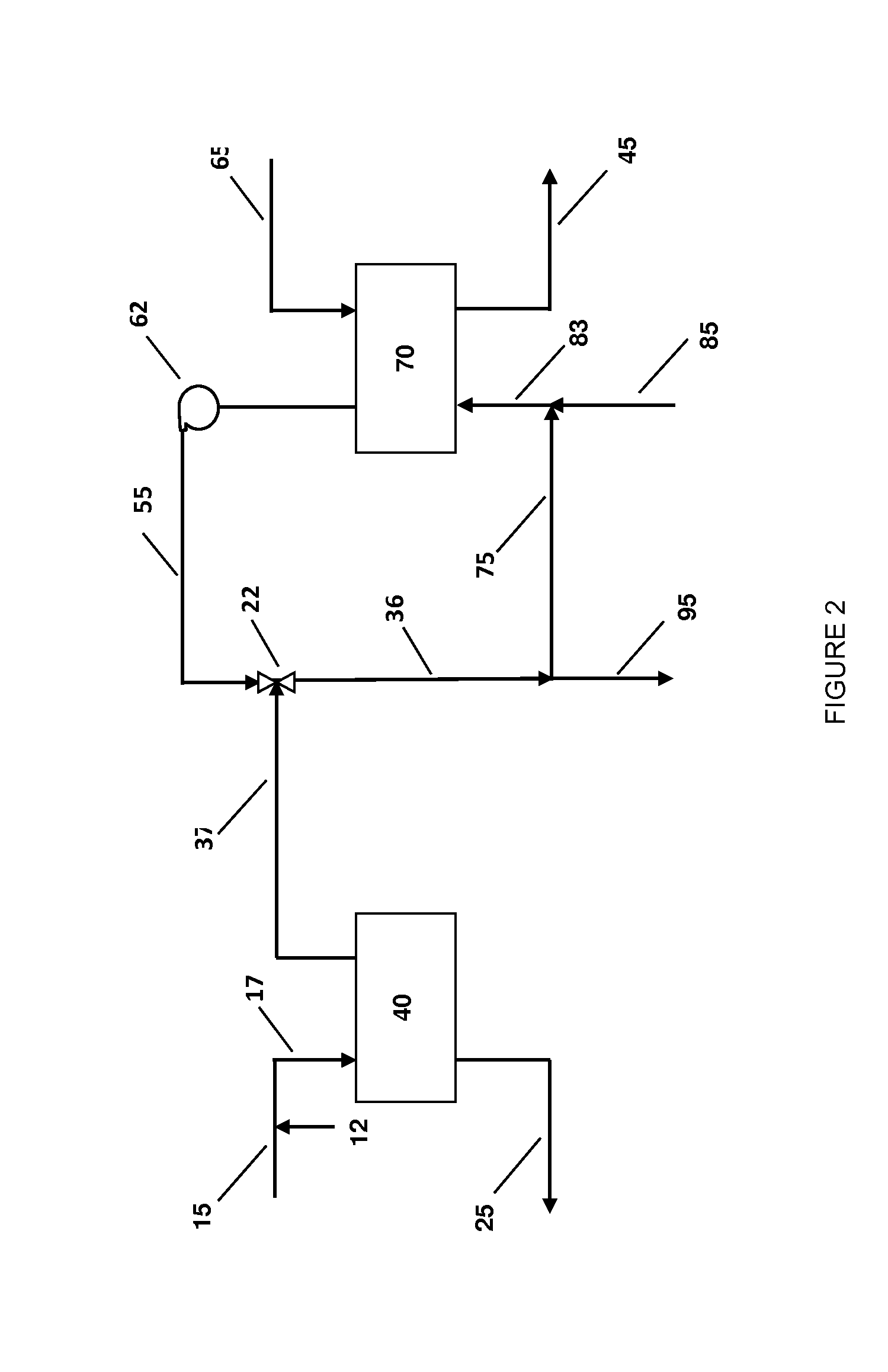
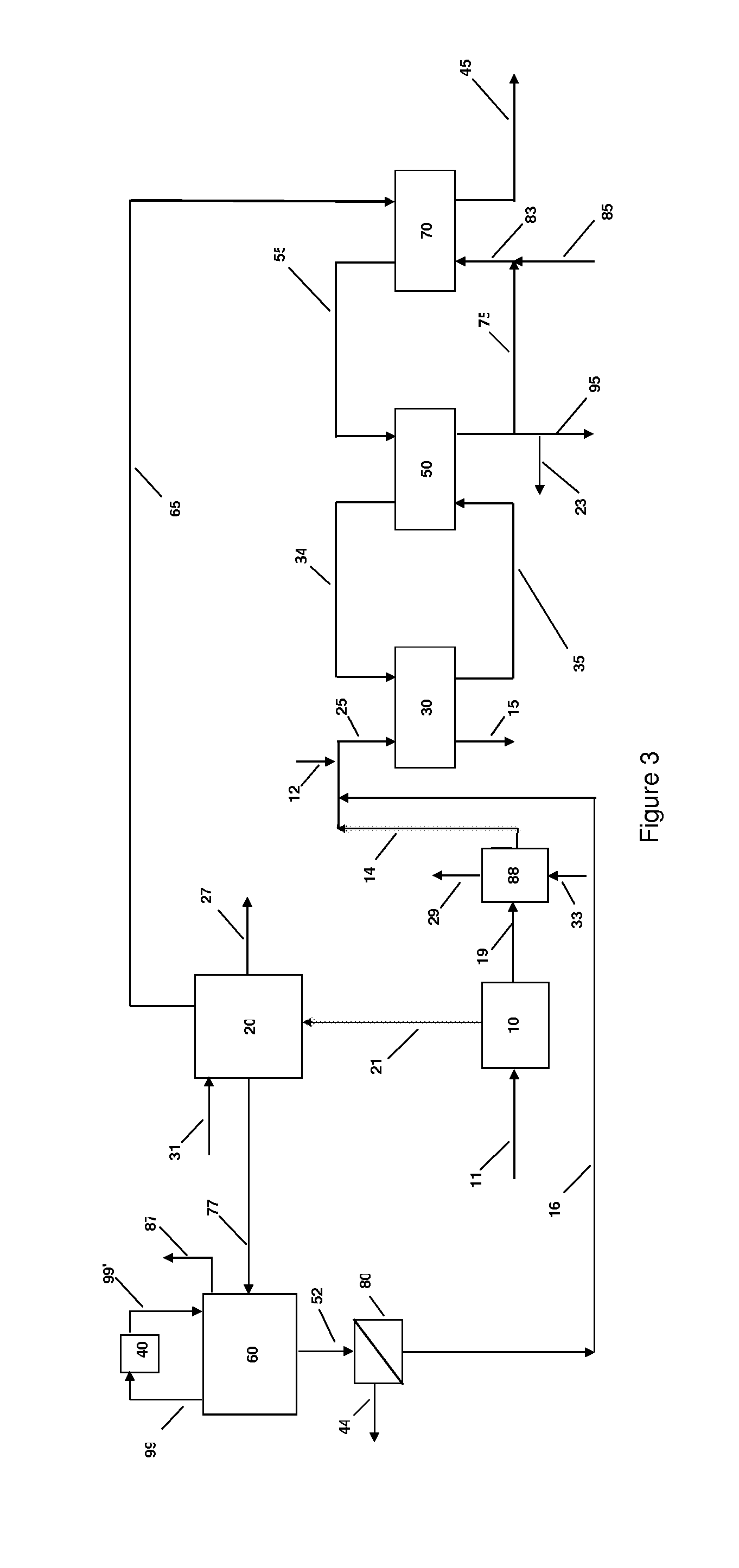
Systems and methods for integrated ammonia-urea process
Systems and methods for producing urea are provided. A method for producing urea can include exchanging heat from a syngas comprising hydrogen and carbon dioxide to a urea solution comprising urea and ammonium carbamate. The heat transferred can be sufficient to decompose at least a portion of the ammonium carbamate. In one or more embodiments, the syngas can be reacted with liquid ammonia to provide a carbon dioxide lean syngas and an ammonium carbamate solution. The ammonium carbamate solution can be heated to a temperature of about 180° C. or more. At least a portion of the ammonium carbamate in the heated ammonium carbamate solution can be dehydrated to provide the urea solution.
Owner:KELLOGG BROWN & ROOT LLC
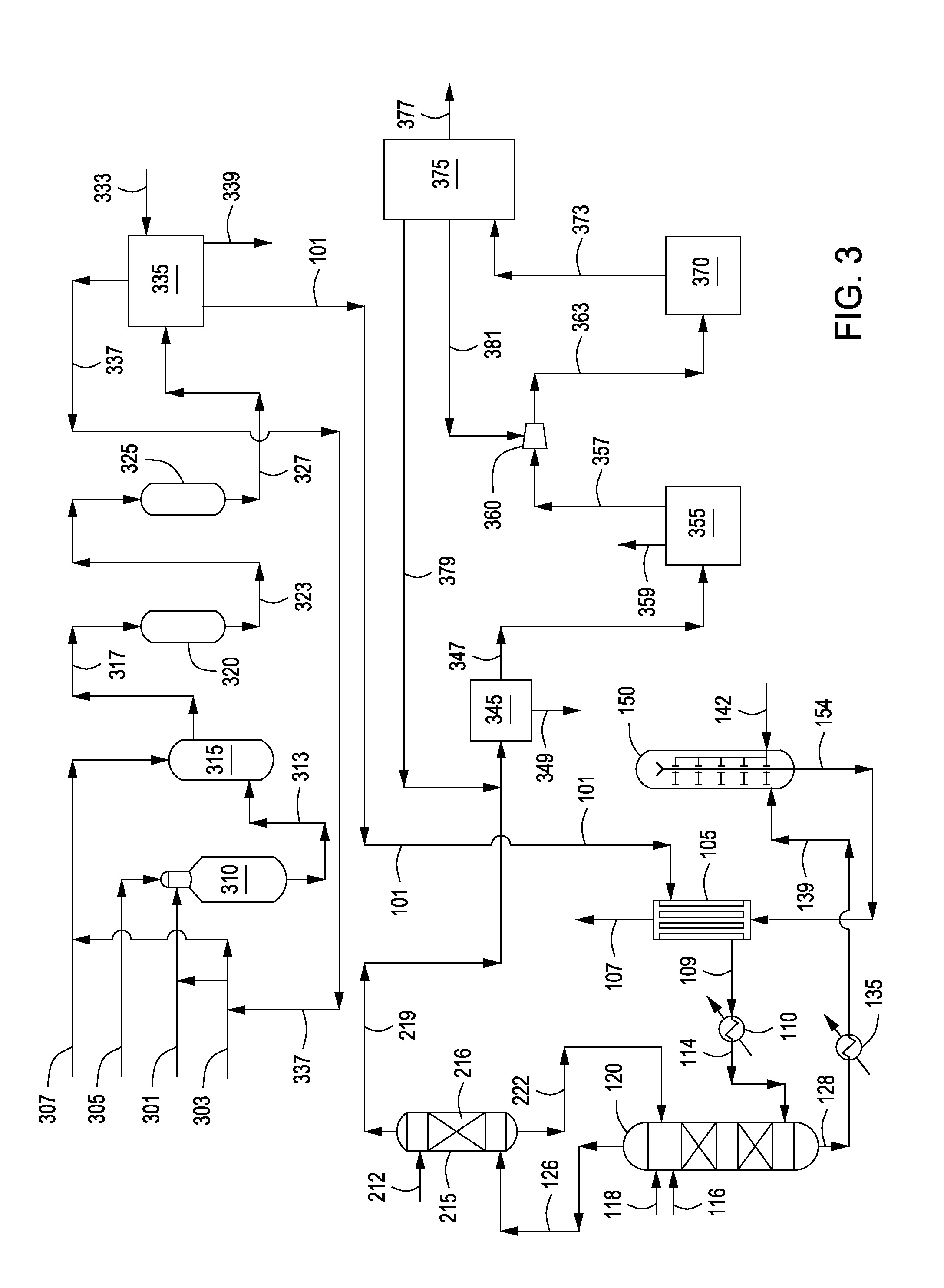
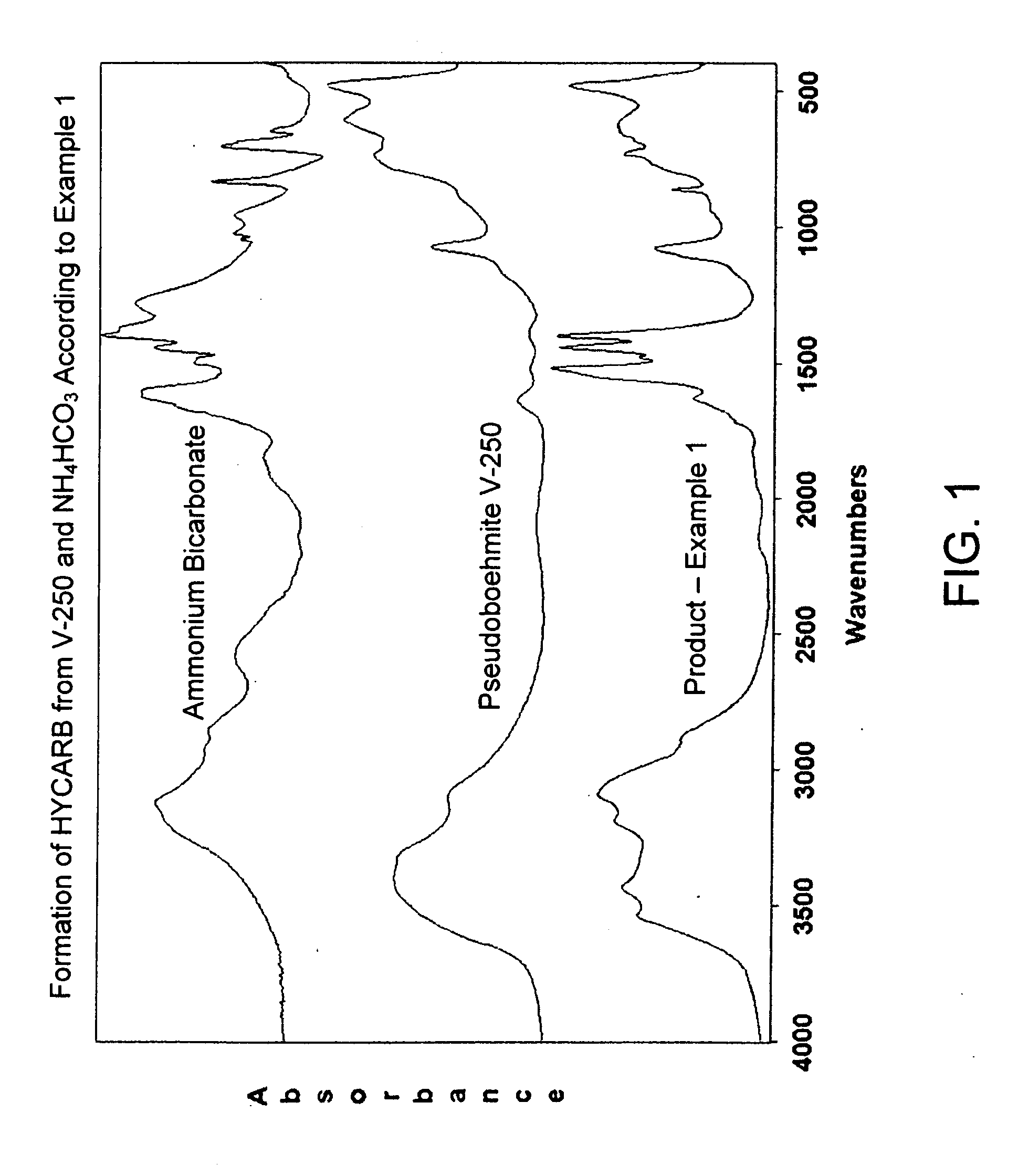
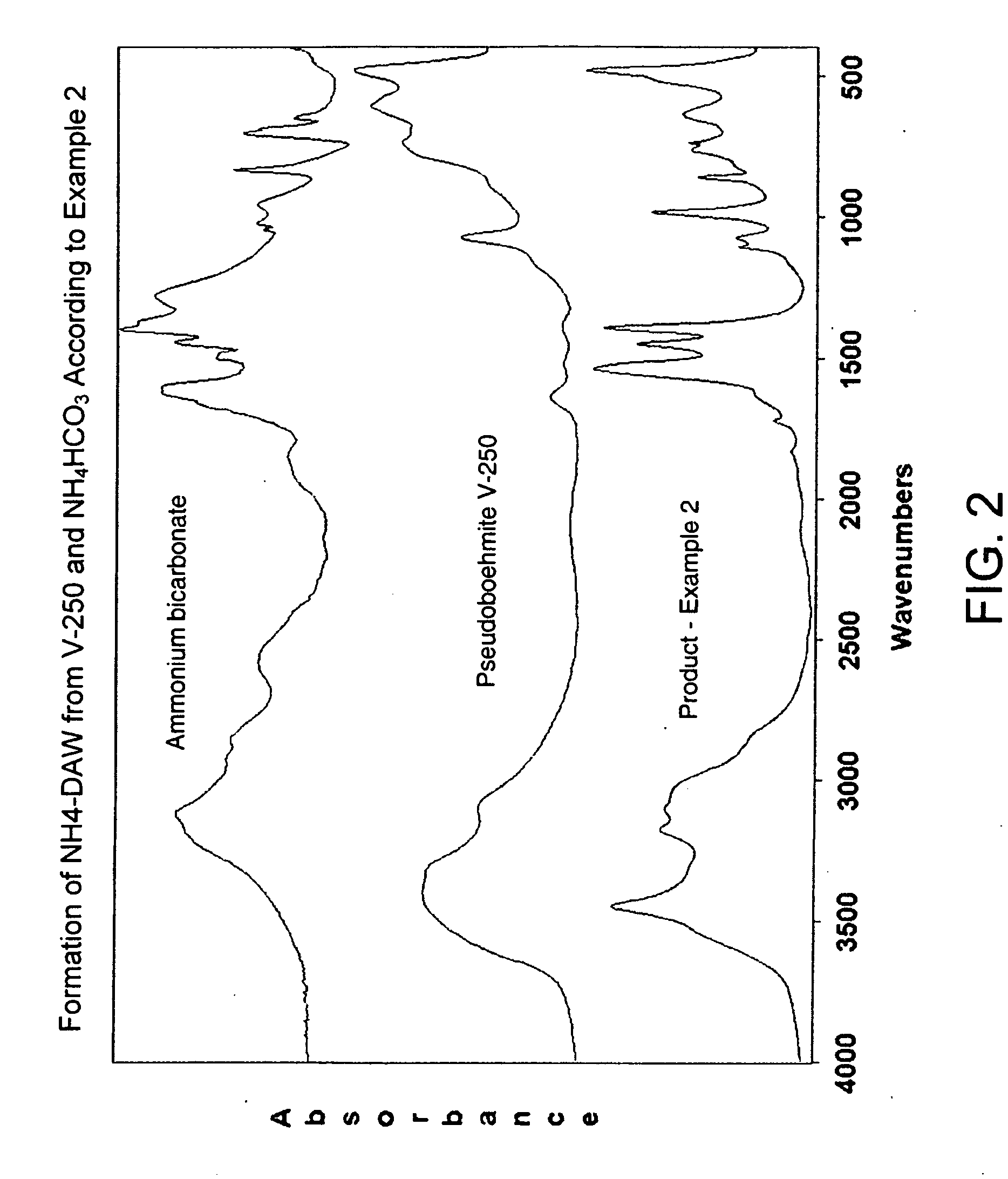
Process for Conversion of Aluminum Oxide Hydroxide
ActiveUS20100148116A1Other chemical processesAmmonium carbonates/bicarbonatesAluminium hydroxideAluminum oxide hydroxide
The present invention is a process for the conversion of aluminum oxide hydroxide (AlOOH) to aluminum oxide. About 30 to 70 wt-% of AlOOH, about 30 to 70 wt-% ammonium hydrogencarbonate NH4HCO3 and 0 to 20 wt-% water are combined to produce a mixture. This mixture is then cured at a temperature from about 30° to about 90° C. to convert at least 5% of the AlOOH to a ammonium hydroxycarbonate (dawsonite-type) intermediate and then the dawsonite-type intermediate is decomposed at a temperature from about 130° to 320° C. to produce aluminum oxide. The aluminum oxide can be further calcined at 500° to 800° C. to produce a gamma-theta phase alumina.
Owner:UOP LLC
Ammonium recovery methods
ActiveUS20130029405A1Increase energy densityIncrease volumeMicroorganismsDispersed particle separationWater useRecovery method
The methods are utilized to recover ammonium from waste water using CO2 acidified absorption water. The process is particularly suited for utilization of cellular matter and a CO2 rich tail gas from a syngas fermentation process and derives significant benefit from the recovery of ammonium bicarbonate and ammonium carbonate. Ammonia and ammonium are recovered from the treatment of the syngas as an ammonium rich solution, at least a portion of which is recycled to the fermentation zone to aid in the production of liquid products. A carbon dioxide rich gas produced by fermentation is used to capture the ammonia and ammonium, forming the ammonium rich solution.
Owner:SYNATA BIO INC
Air battery catalyst and air battery using the same
ActiveUS20120003548A1High potential in rechargingImprove discharge characteristicsFuel and primary cellsFuel and secondary cellsNiobiumElectrical battery
Catalysts are provided which can catalyze both the oxygen reduction during the discharge of a secondary air battery and the oxygen production in the recharging of the battery and which are stable at a high potential in the recharging. The invention has been accomplished based on the finding that a catalyst including an oxycarbonitride of a specific transition metal selected from, for example, titanium, zirconium, hafnium, vanadium, niobium and tantalum can catalyze both the oxygen reduction during the discharge of a secondary air battery and the oxygen production in the recharging of the battery and is also stable at a high potential in the recharging.
Owner:SHOWA DENKO KK
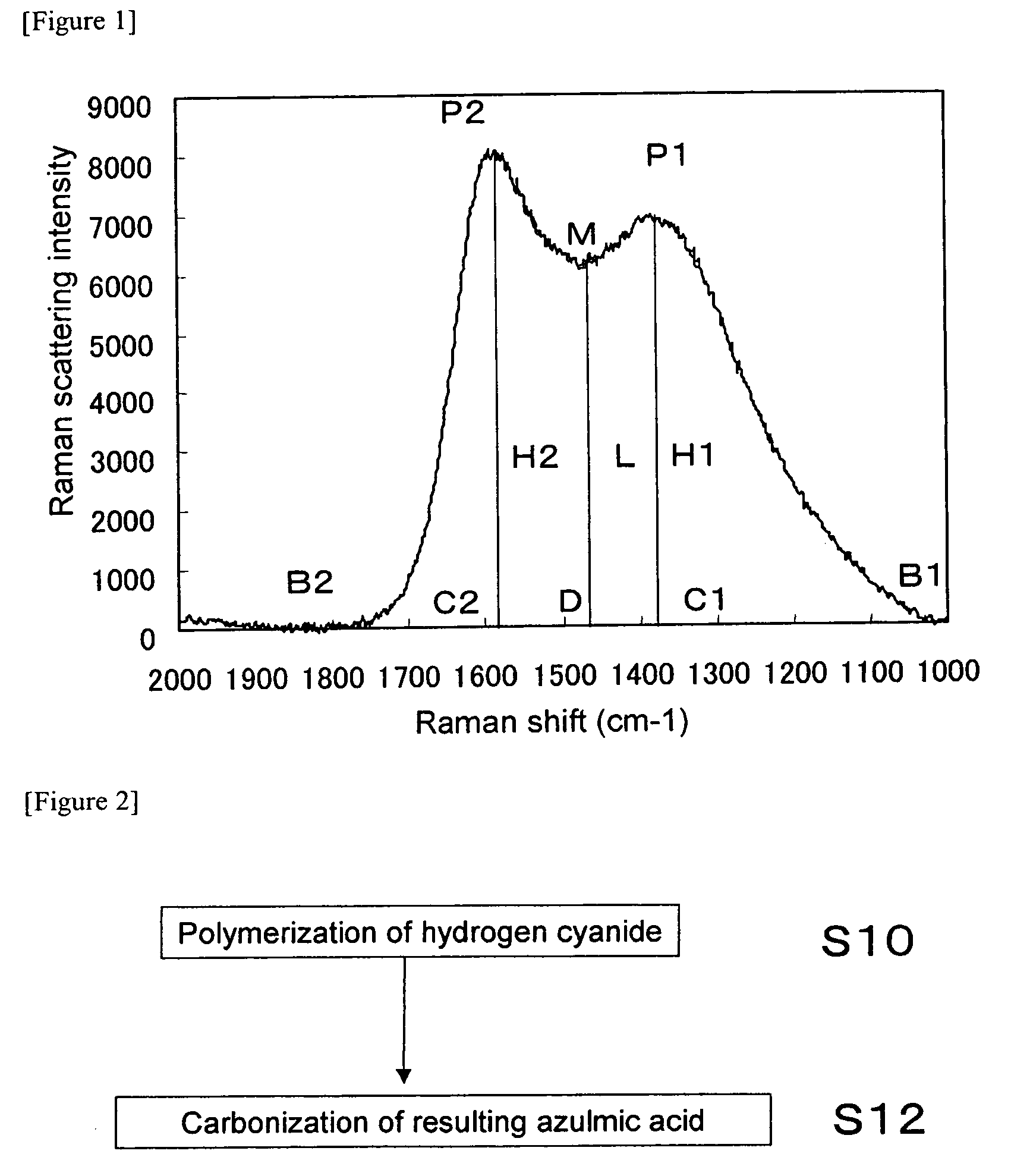
Nitrogen-Containing Carbon Material and Method of Producing the Same
ActiveUS20090112020A1Efficient productionEasy to produceOrganic chemistryHybrid capacitor electrodesX-rayLaser raman
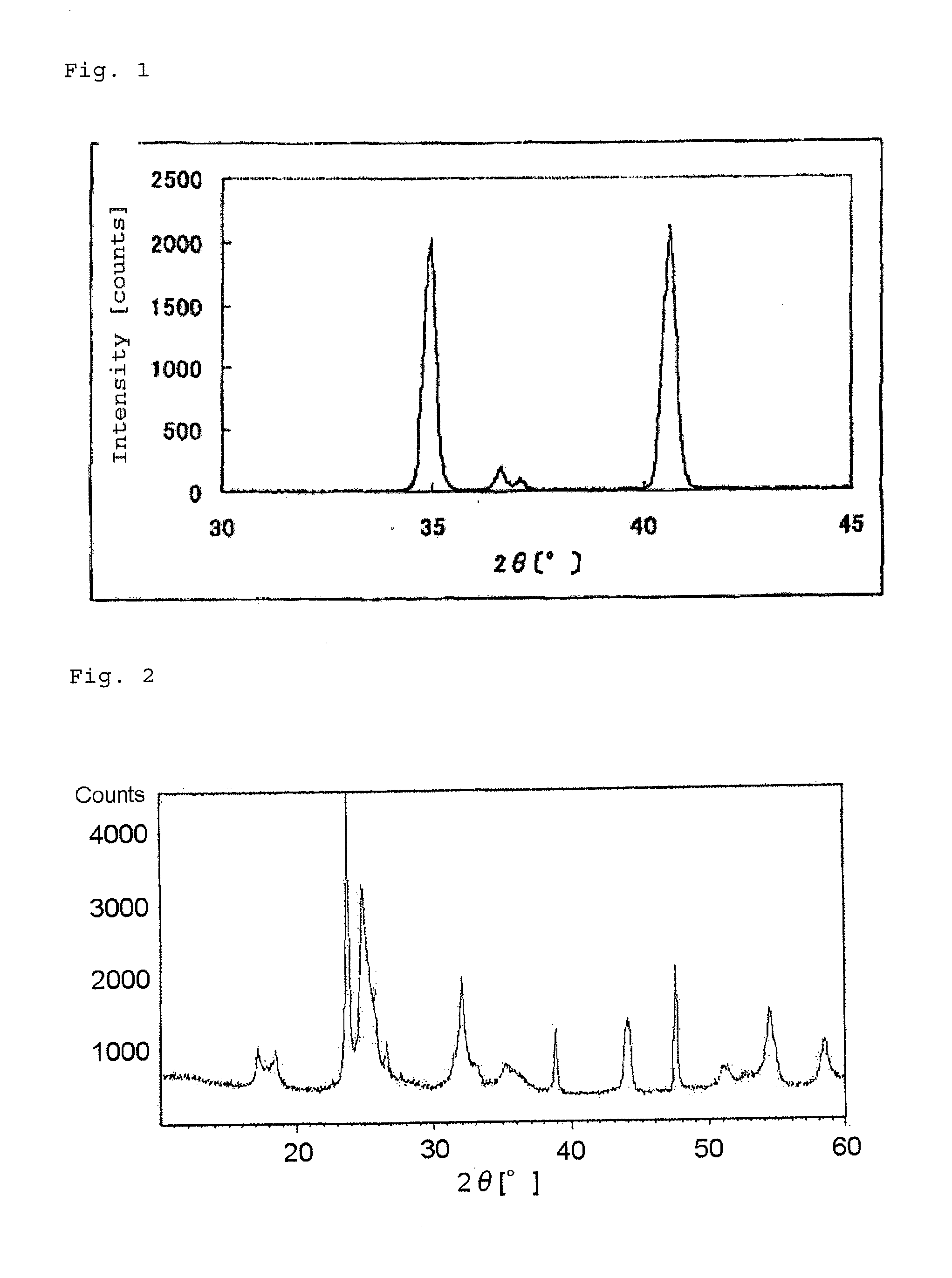
The present invention provides a nitrogen-containing carbon material characterized in that it satisfies a specific relational expression between the number ratio of nitrogen atoms to carbon atoms and the number ratio of hydrogen atoms to carbon atoms and has peaks in specific regions in the X-ray diffraction and in the laser Raman spectrum. The nitrogen-containing carbon material of the present invention can be produced by carbonizing azulmic acid in an inert gas atmosphere, and it is useful as an electrode material or the like because it has a high nitrogen content and a low hydrogen content.
Owner:ASAHI KASEI CHEM CORP
Process for production and use of carbonitride mixture particles or oxycarbonitride mixture particles
InactiveUS20110183234A1Uniform particle size distributionMaintain good propertiesMaterial nanotechnologyCatalyst protectionLaser lightNitrogen oxide
The invention has an object of providing catalysts that are not corroded in acidic electrolytes or at high potential, have excellent durability and show high oxygen reducing ability. An aspect of the invention is directed to a process wherein metal carbonitride mixture particles or metal oxycarbonitride mixture particles are produced from an organometallic compound of a Group IV or V transition metal, a metal salt of a Group IV or V transition metal, or a mixture of these compounds using laser light as a light source.
Owner:SHOWA DENKO KK
Catalyst, production process therefor and use thereof
InactiveUS20110053040A1No corrosionReduce capacityPhysical/chemical process catalystsActive material electrodesIndiumCerium
Catalysts of the invention are not corroded in acidic electrolytes or at high potential and have excellent durability and high oxygen reducing ability. A catalyst includes a metal oxycarbonitride containing niobium and at least one metal M selected from the group consisting of tin, indium, platinum, tantalum, zirconium, copper, iron, tungsten, chromium, molybdenum, hafnium, titanium, vanadium, cobalt, manganese, cerium, mercury, plutonium, gold, silver, iridium, palladium, yttrium, ruthenium, lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium and nickel.
Owner:SHOWA DENKO KK
Coated Member
ActiveUS20120128971A1Improve adhesionExcellent in wear resistance and fracture resistance and oxidation resistancePigmenting treatmentOther chemical processesX-rayGroup 6 element
A coated member includes a base material and a coating film formed on the surface thereof. At least one layer in the coating film is a hard film of a cubic metal compound including at least one element selected from the group consisting of the group 4 elements (Ti, Zr, Hf, etc.), group 5 elements (V, Nb, Ta, etc.) and group 6 elements (Cr, Mo, W, etc.) of the periodic table, Al, Si, B, Y and Mn together with at least one element selected from the group consisting of C, N and O. In the pole figure for the face (111) of the hard film, the X-ray intensity distribution in the α-axis shows the maximum intensity in the α-angle range of 50-65°. In the pole figure for the face (200), the X-ray intensity distribution in the α-axis shows the maximum intensity in the α-angle range of 60-80°.
Owner:TUNGALOY CORP
Process for producing a fuel cell electrode catalyst, fuel cell electrode catalyst and use thereof
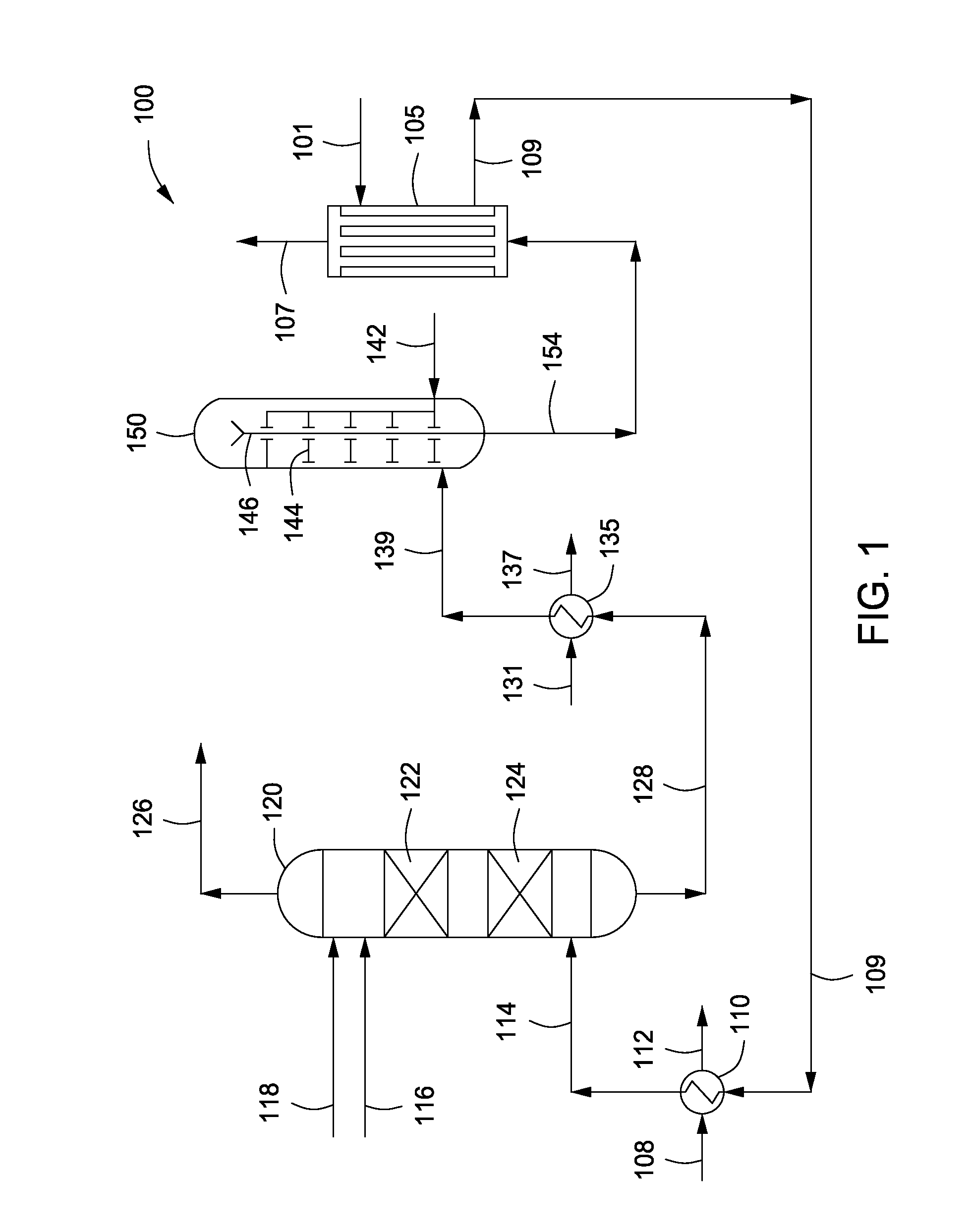
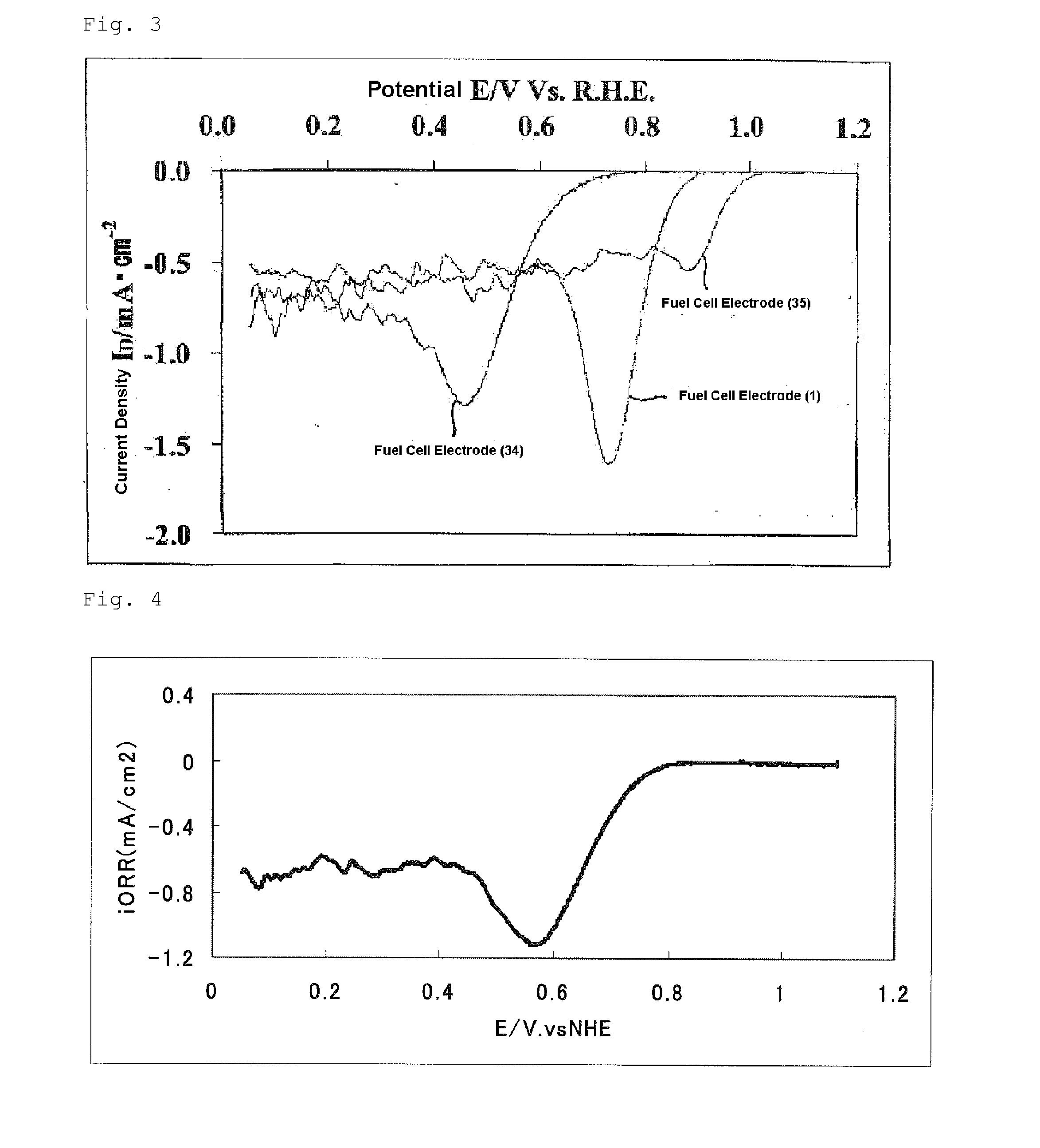
InactiveUS20140170528A1High catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsActive material electrodesNiobiumCobalt
Provided is a process for producing a fuel cell electrode catalyst with high catalytic activity that is alternative to a noble metal catalyst, through a heat treatment at a relatively low temperature. A process for producing a fuel cell electrode catalyst includes a step (I) of obtaining a catalyst precursor, including a step (Ia) of mixing at least a metal compound (1), a nitrogen-containing organic compound (2), and a fluorine-containing compound (3), and a step (II) of heat-treating the catalyst precursor at a temperature of 500 to 1300° C. to obtain an electrode catalyst, a portion or the entirety of the metal compound (1) being a compound containing an atom of a metal element M1 selected from the group consisting of iron, cobalt, chromium, nickel, copper, zinc, titanium, niobium and zirconium, and at least one of the compounds (1), (2) and (3) containing an oxygen atom.
Owner:SHOWA DENKO KK
Systems and methods for integrated ammonia-urea process
Systems and methods for producing urea are provided. A method for producing urea can include exchanging heat from a syngas comprising hydrogen and carbon dioxide to a urea solution comprising urea and ammonium carbamate. The heat transferred can be sufficient to decompose at least a portion of the ammonium carbamate. In one or more embodiments, the syngas can be reacted with liquid ammonia to provide a carbon dioxide lean syngas and an ammonium carbamate solution. The ammonium carbamate solution can be heated to a temperature of about 180° C. or more. At least a portion of the ammonium carbamate in the heated ammonium carbamate solution can be dehydrated to provide the urea solution.
Owner:KELLOGG BROWN & ROOT LLC
Compositions for stabilizing chlorinated water to sunlight decomposition, and methods of preparation thereof
ActiveUS7728132B2Process stabilityLow viscosityOrganic chemistryOther chemical processesDecompositionSlurry
The invention includes a composition for stabilizing chlorinated water to sunlight decomposition, and methods of preparing compositions. The composition is a slurry composition of a monoalkali metal cyanurate, of low viscosity. Two methods of preparing the slurry are described, in which cyanuric acid or cyanuric acid wetcake is mixed with a monoalkali metal base. One method dry blends cyanuric acid or cyanuric acid wetcake with a monoalkali metal base. The invention also describes a method of preparing a dry, solid monoalkali metal cyanurate.
Owner:ENVIRO TECH CHEM SERVICES
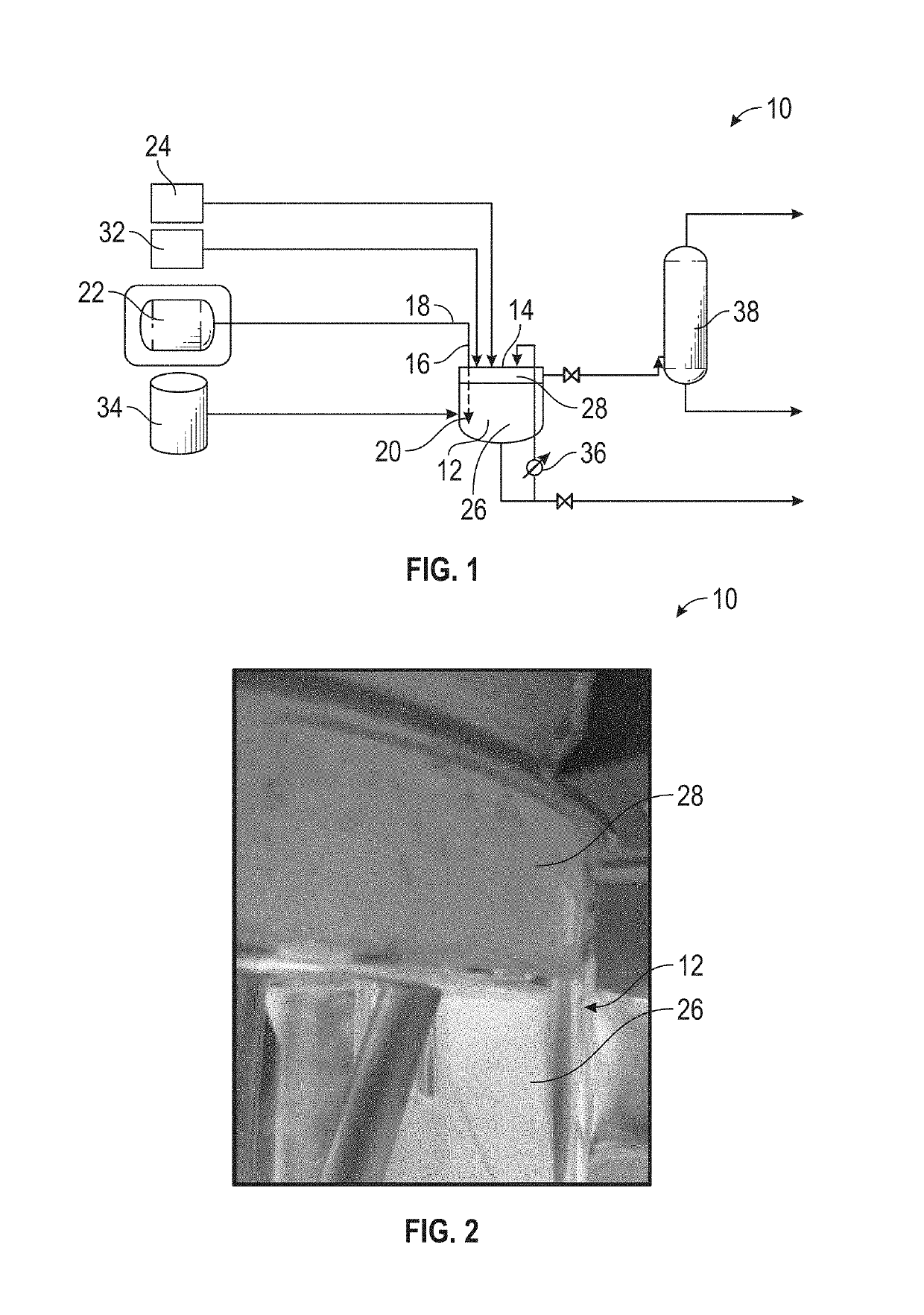
Apparatus for Processing Highly Corrosive Agents
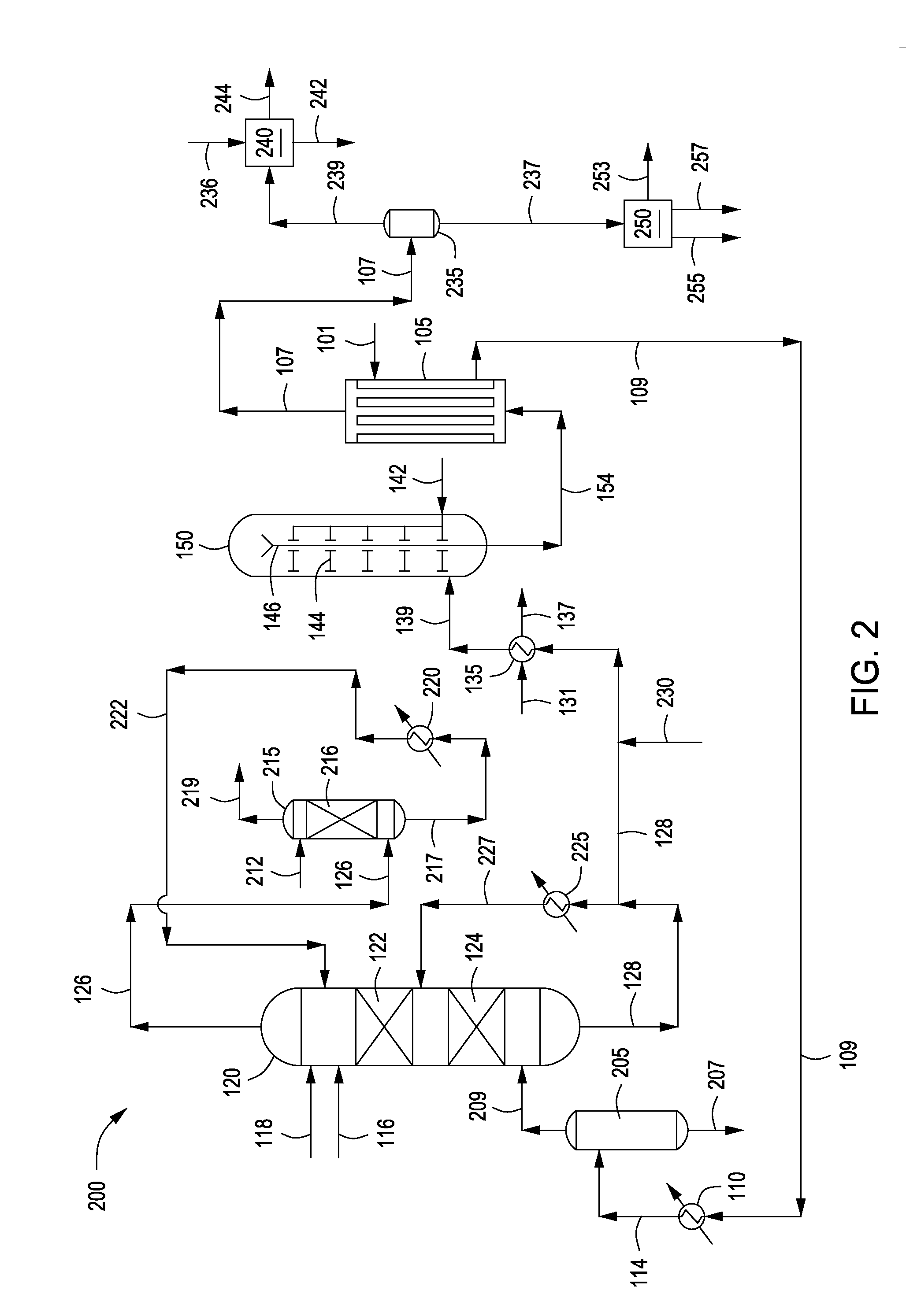
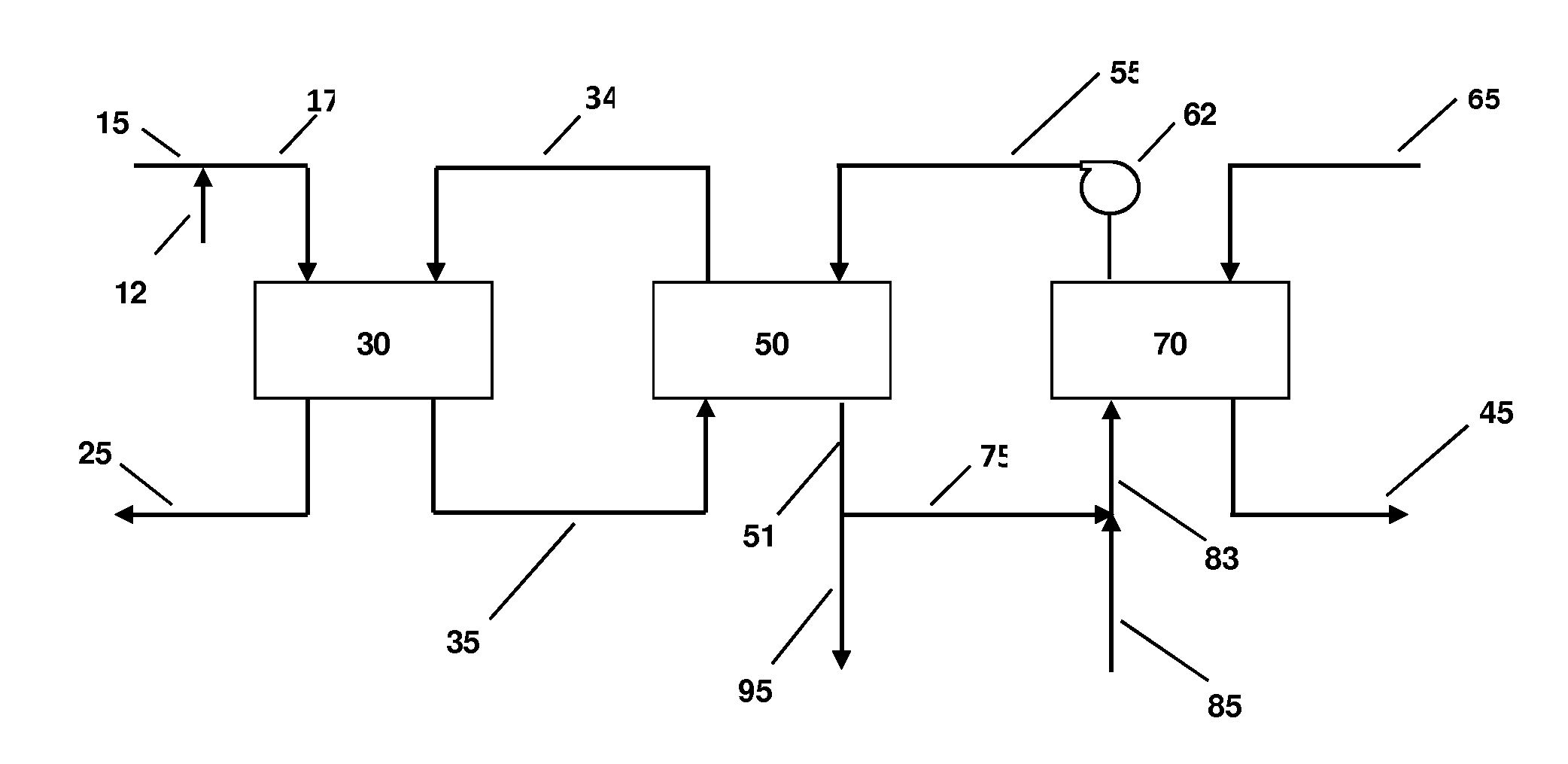
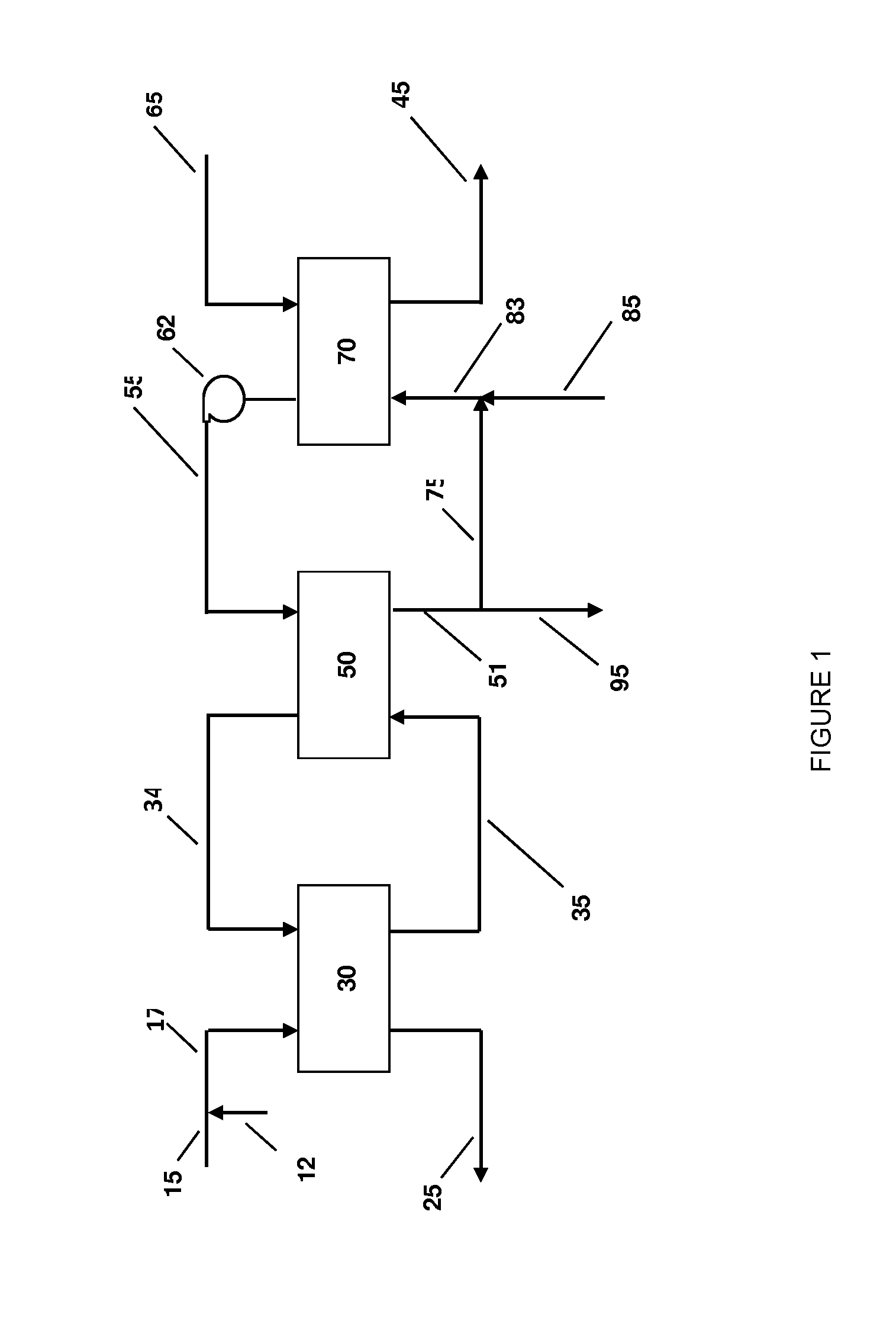
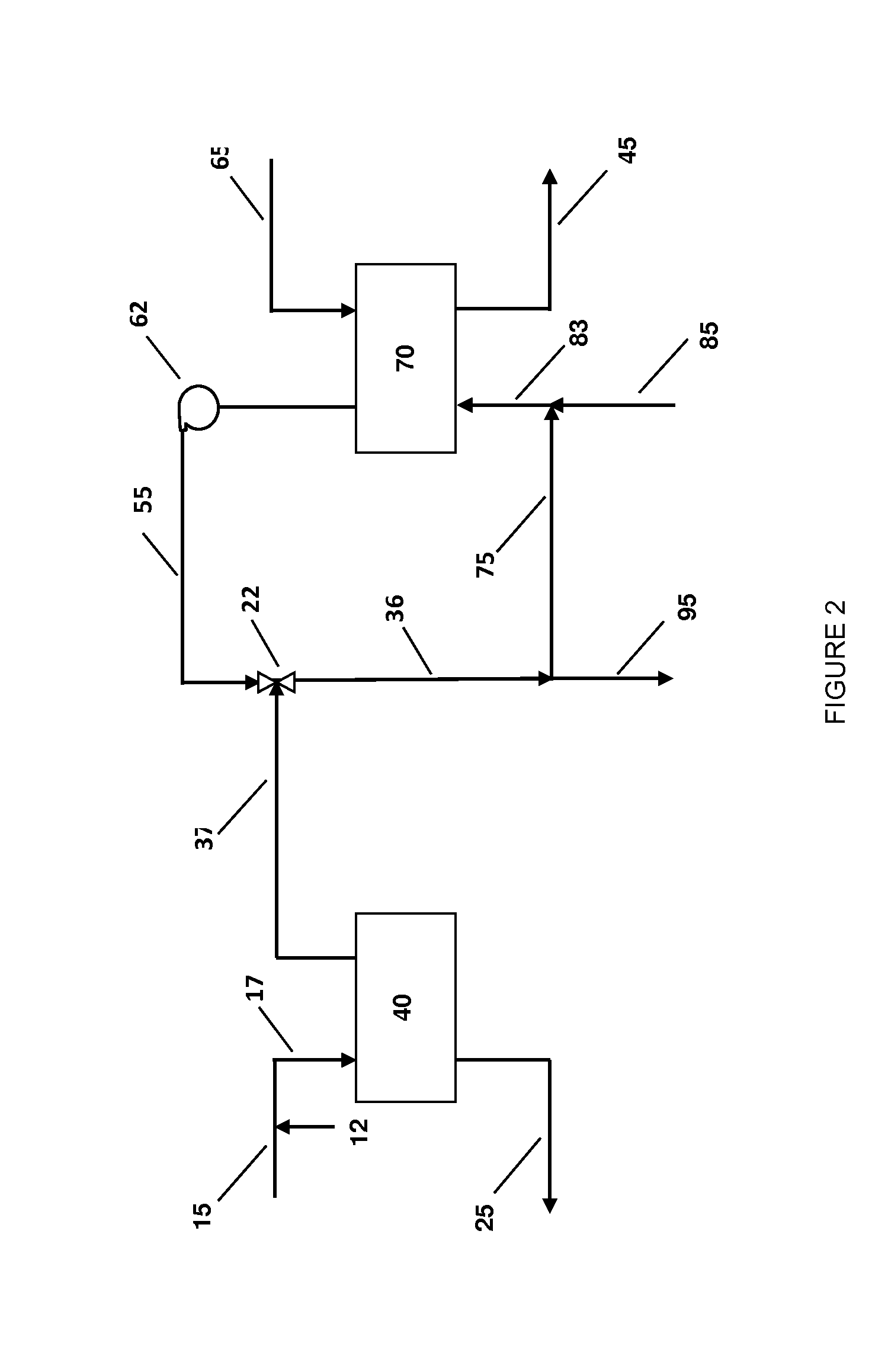
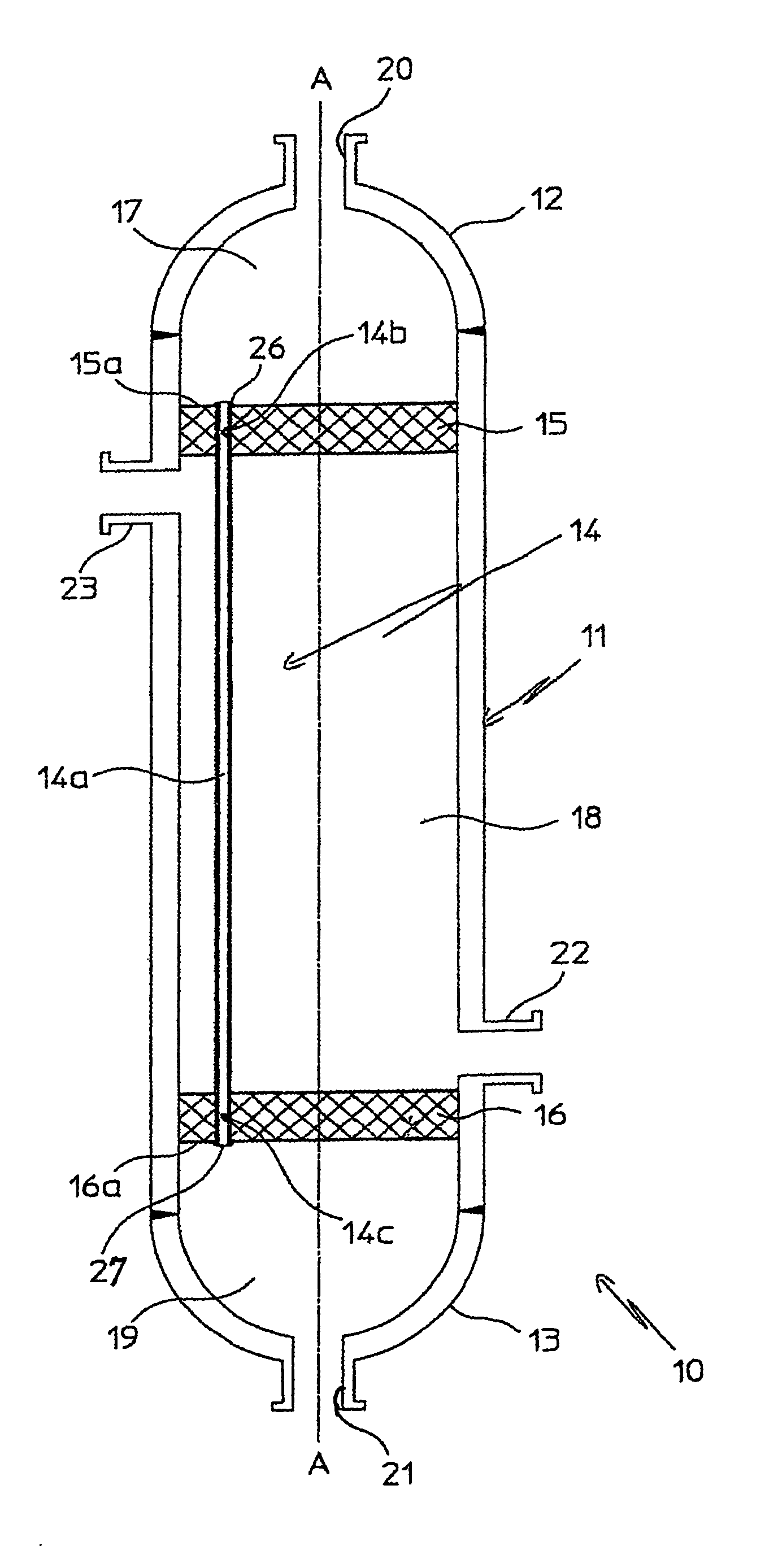
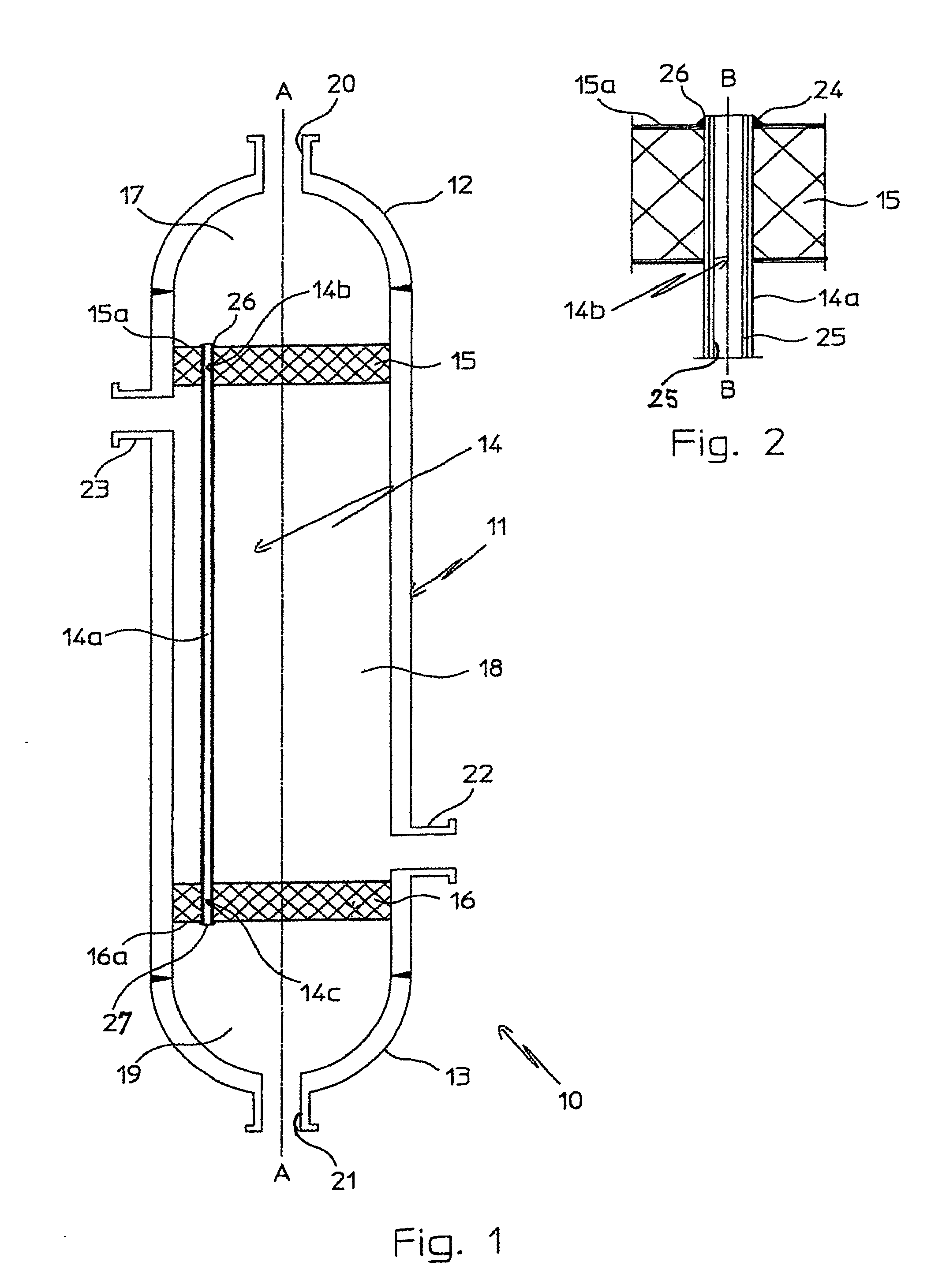
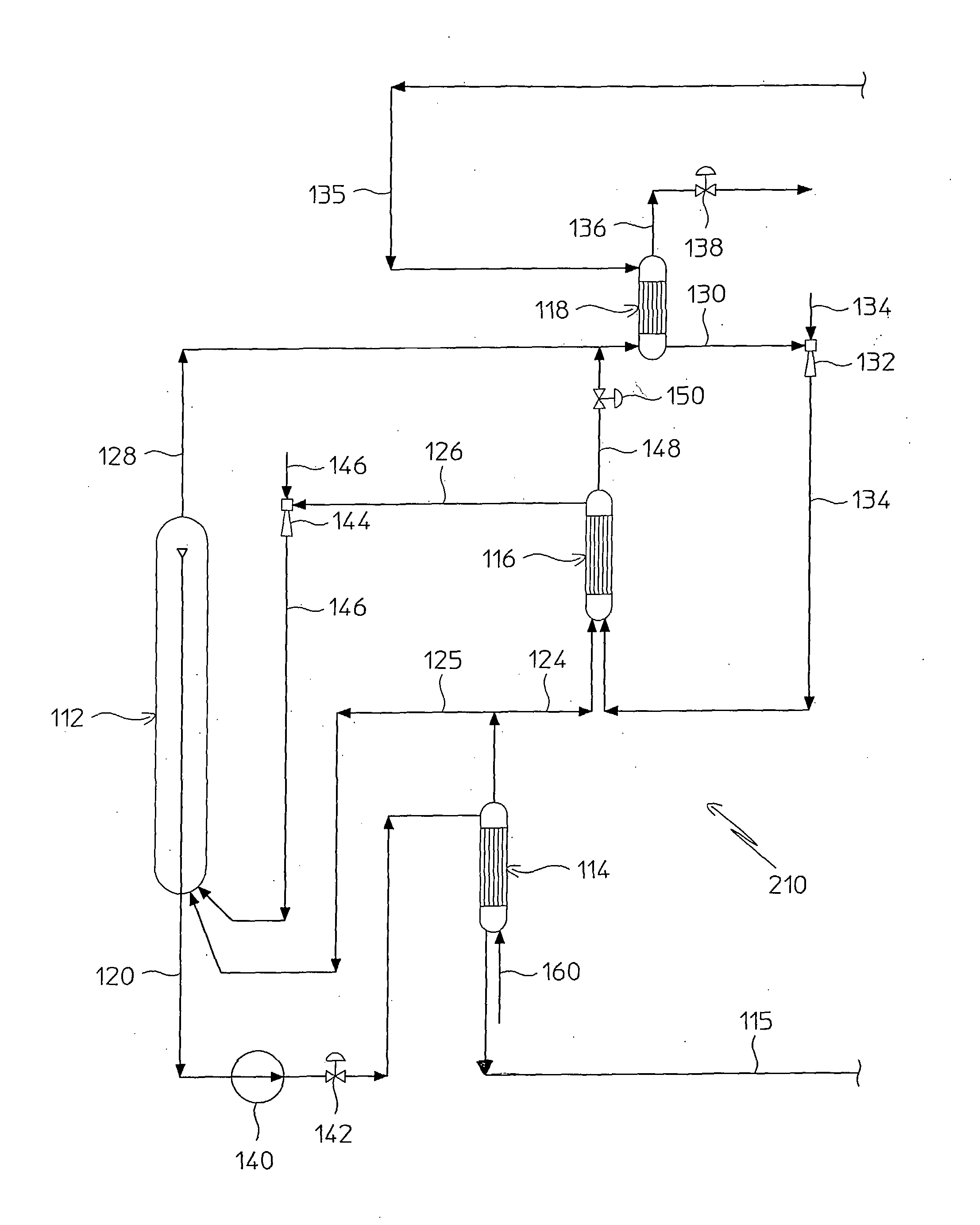
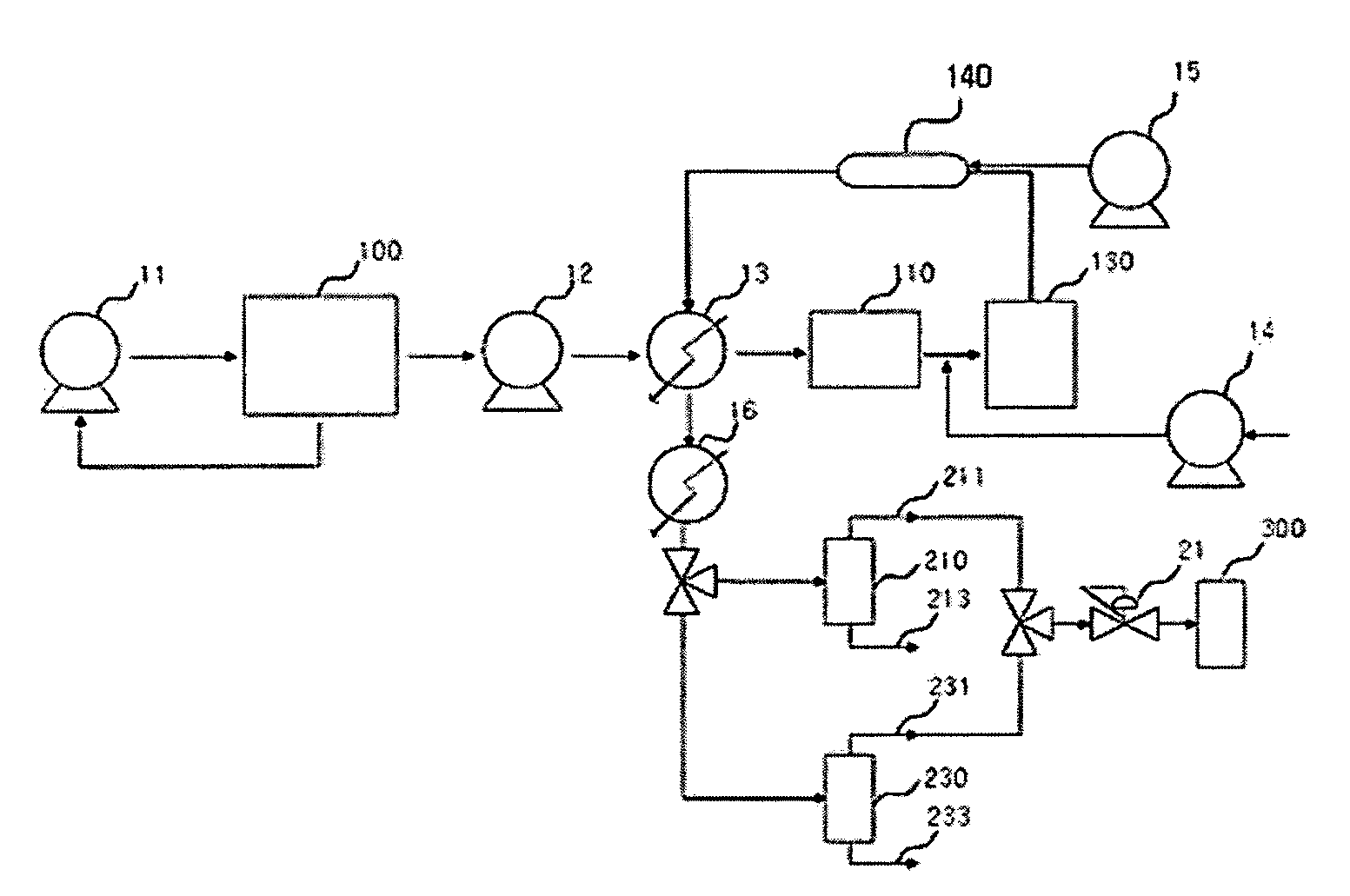
InactiveUS20070235171A1Easily realizedAvoid crackingCarbamic acidGas-gas reaction processesEngineeringHeat exchanger
An apparatus for treating highly corrosive agents comprises a tube bundle (14) heat exchanger (10), structured to carry out a heat exchange between two fluids one of which is highly corrosive and flowing inside of said tube bundle (14).
Owner:CASALE SA
Process for producing oxygen reducing catalyst and uses thereof
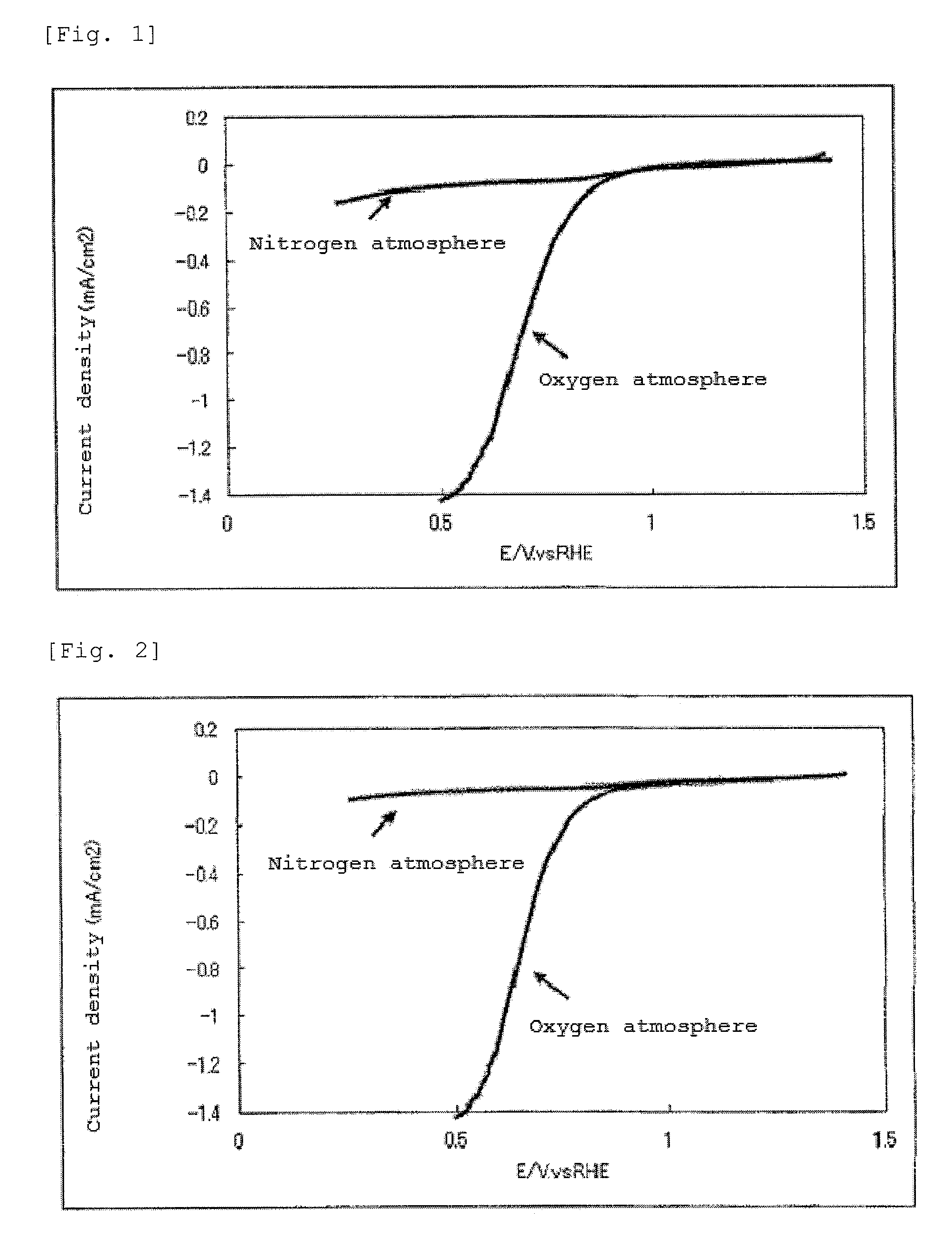
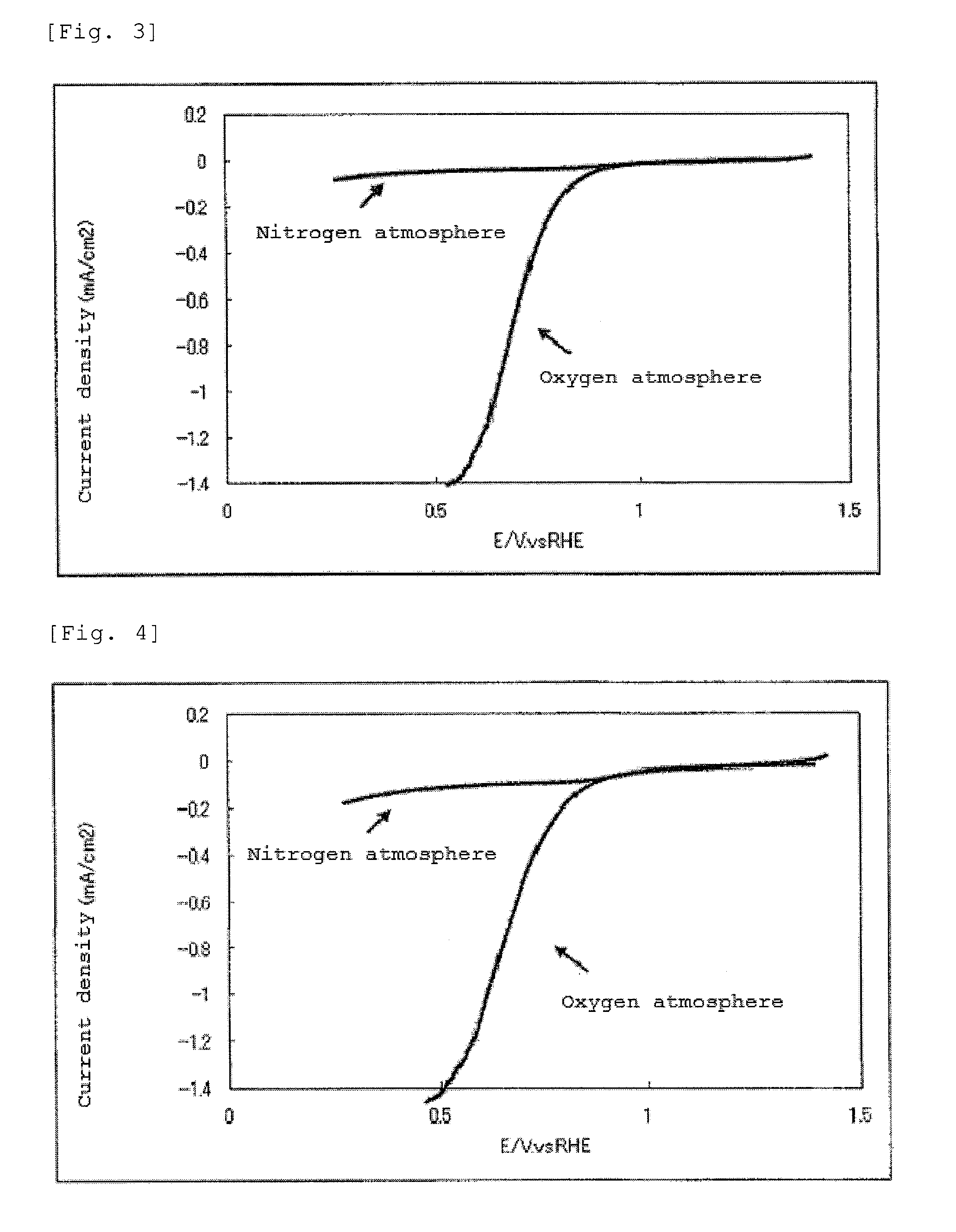
InactiveUS20140178790A1Reduce capacityPhysical/chemical process catalystsActive material electrodesFuel cellsNitrogen
A process for producing an oxygen reducing catalyst including a step of heat-treating, in a non-oxidizing atmosphere, a catalyst precursor including a compound (i) supplying a carbon element and a nitrogen element by heating in a non-oxidizing atmosphere, and a compound (ii) containing at least one element of iron and cobalt. Also disclosed is an oxygen reducing catalyst, a fuel cell catalyst layer including the oxygen reducing catalyst, an electrode including the fuel cell catalyst layer, a membrane-electrode assembly including the electrode and a fuel cell including the membrane-electrode assembly.
Owner:SHOWA DENKO KK
Catalyst, production process therefor and use thereof
InactiveUS8496903B2Raise the potentialImprove performancePhysical/chemical process catalystsActive material electrodesNiobiumAcid electrolyte
Catalysts of the invention are not corroded in acidic electrolytes or at high potential and have excellent durability and high oxygen reducing ability. The catalysts include a niobium oxycarbonitride represented by a compositional formula NbCxNyOz (wherein x, y and z represent a ratio of the numbers of the atoms, 0.05≦x<0.7, 0.01≦y<0.7, 0.4≦z<2.5, 1.0<x+y+z<2.56, and 4.0≦4x+3y+2z).
Owner:SHOWA DENKO KK
Catalyst carrier, catalyst and process for producing the same
InactiveUS8541334B2Improve heat resistanceHigh catalytic ability without increasing the specific surface areaCell electrodesCatalyst activation/preparationIndiumCerium
The present invention provides a catalyst carrier having excellent durability and capable of attaining high catalytic ability without increasing the specific surface area thereof, and a catalyst obtainable by using the catalyst carrier. The catalyst carrier of the present invention comprises a metal oxycarbonitride, preferably the metal contained in the metal oxycarbonitride comprises at least one selected from the group consisting of niobium, tin, indium, platinum, tantalum, zirconium, copper, iron, tungsten, chromium, molybdenum, hafnium, titanium, vanadium, cobalt, manganese, cerium, mercury, plutonium, gold, silver, iridium, palladium, yttrium, ruthenium, lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, and nickel. Moreover, the catalyst of the present invention comprises the catalyst carrier and a catalyst metal supported on the catalyst carrier.
Owner:SHOWA DENKO KK
Catalyst, production process therefor and use thereof
InactiveUS20110059386A1No corrosionReduce capacityPhysical/chemical process catalystsActive material electrodesNiobiumAcid electrolyte
Catalysts of the invention are not corroded in acidic electrolytes or at high potential and have excellent durability and high oxygen reducing ability. The catalysts include a niobium oxycarbonitride represented by a compositional formula NbCxNyOz (wherein x, y and z represent a ratio of the numbers of the atoms, 0.05≦x<0.7, 0.01≦y<0.7, 0.4≦z<2.5, 1.0<x+y+z<2.56, and 4.0≦4x+3y+2z).
Owner:SHOWA DENKO KK
Strain sensors, methods of making same, and applications of same
InactiveUS9068283B2Improve conductivityMaterial nanotechnologyConductive materialYarnElectrical resistance and conductance
In one aspect, the present invention relates to a layered structure usable in a strain sensor. In one embodiment, the layered structure has a substrate with a first surface and an opposite, second surface defining a body portion therebetween; and a film of carbon nanotubes deposited on the first surface of the substrate, wherein the film of carbon nanotubes is conductive and characterized with an electrical resistance. In one embodiment, the carbon nanotubes are aligned in a preferential direction. In one embodiment, the carbon nanotubes are formed in a yarn such that any mechanical stress increases their electrical response. In one embodiment, the carbon nanotubes are incorporated into a polymeric scaffold that is attached to the surface of the substrate. In one embodiment, the surfaces of the carbon nanotubes are functionalized such that its electrical conductivity is increased.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Method for producing fuel cell catalyst, fuel cell catalyst, and uses thereof
InactiveUS20130115542A1Increased durabilityCatalyst protectionActive material electrodesFuel cellsNitrogen oxide
A method for producing a fuel cell catalyst containing a metal oxycarbonitride, the method including: a step of producing a metal oxycarbonitride by heating a metal carbonitride in an inert gas containing oxygen gas; and a step of bringing the metal oxycarbonitride into contact with an acidic solution.
Owner:SHOWA DENKO KK
Catalyst, production process therefor and use thereof
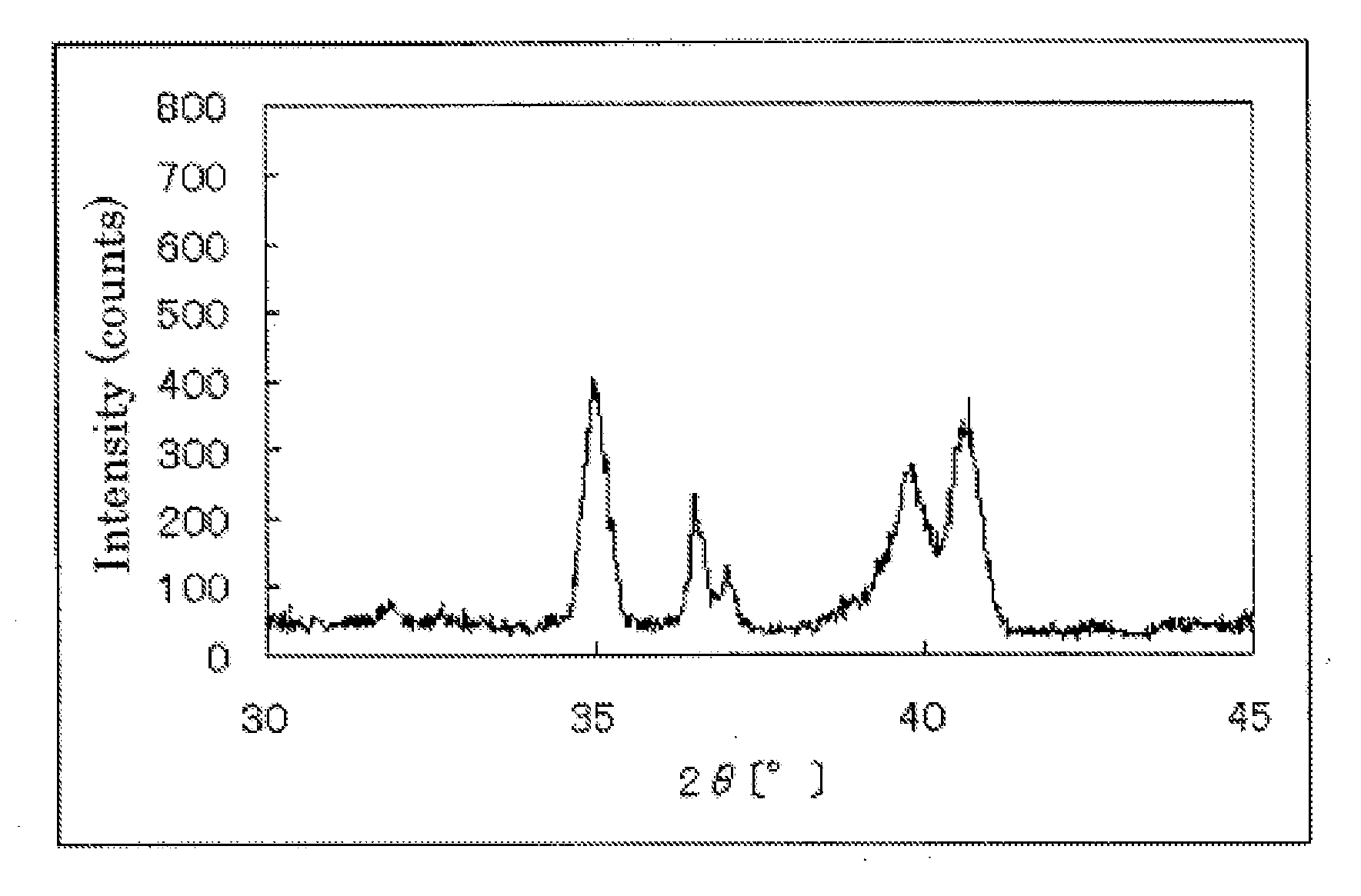
InactiveUS20110189583A1Raise the potentialImprove performanceCatalyst protectionFinal product manufactureCrystallographyNiobium
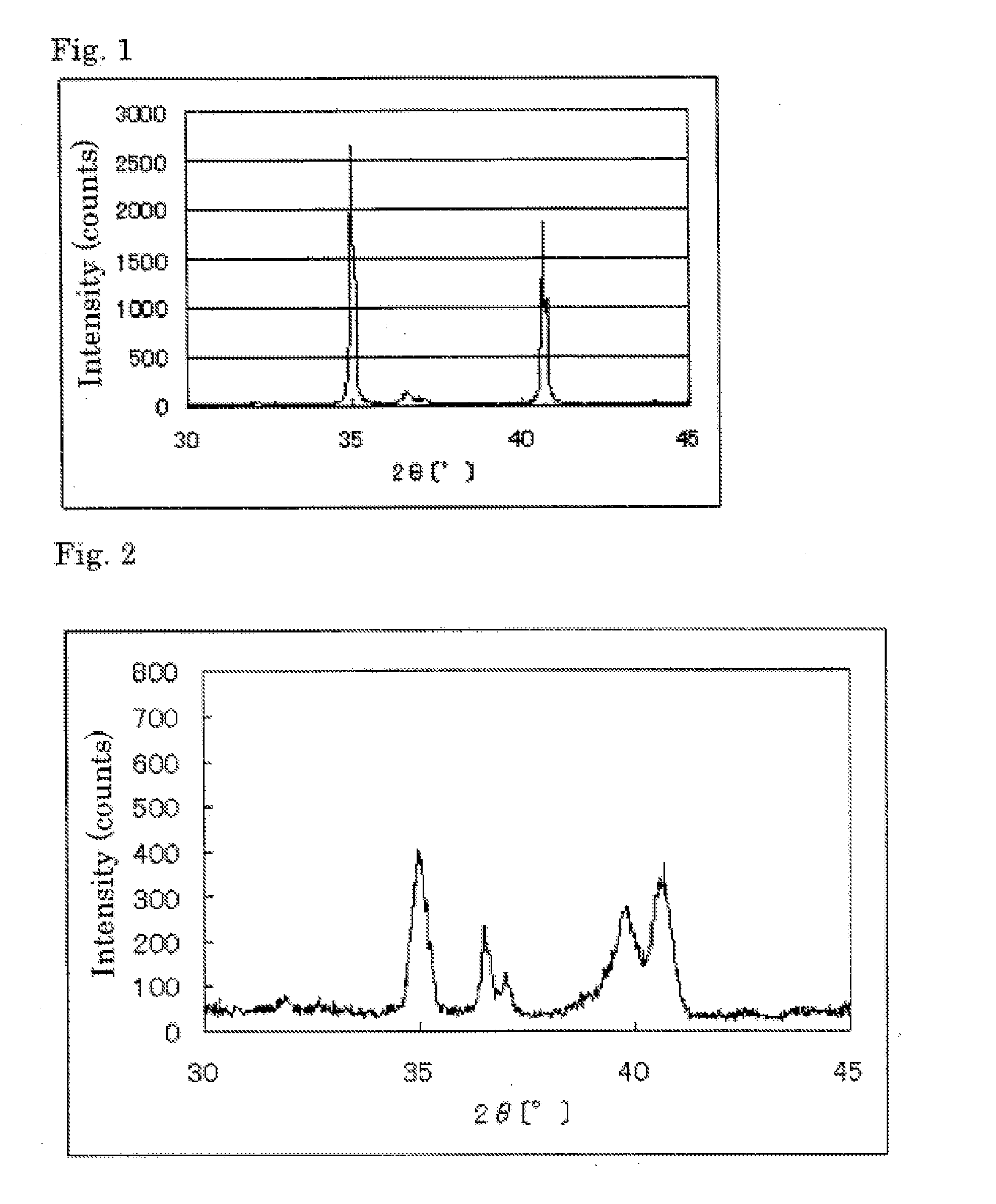
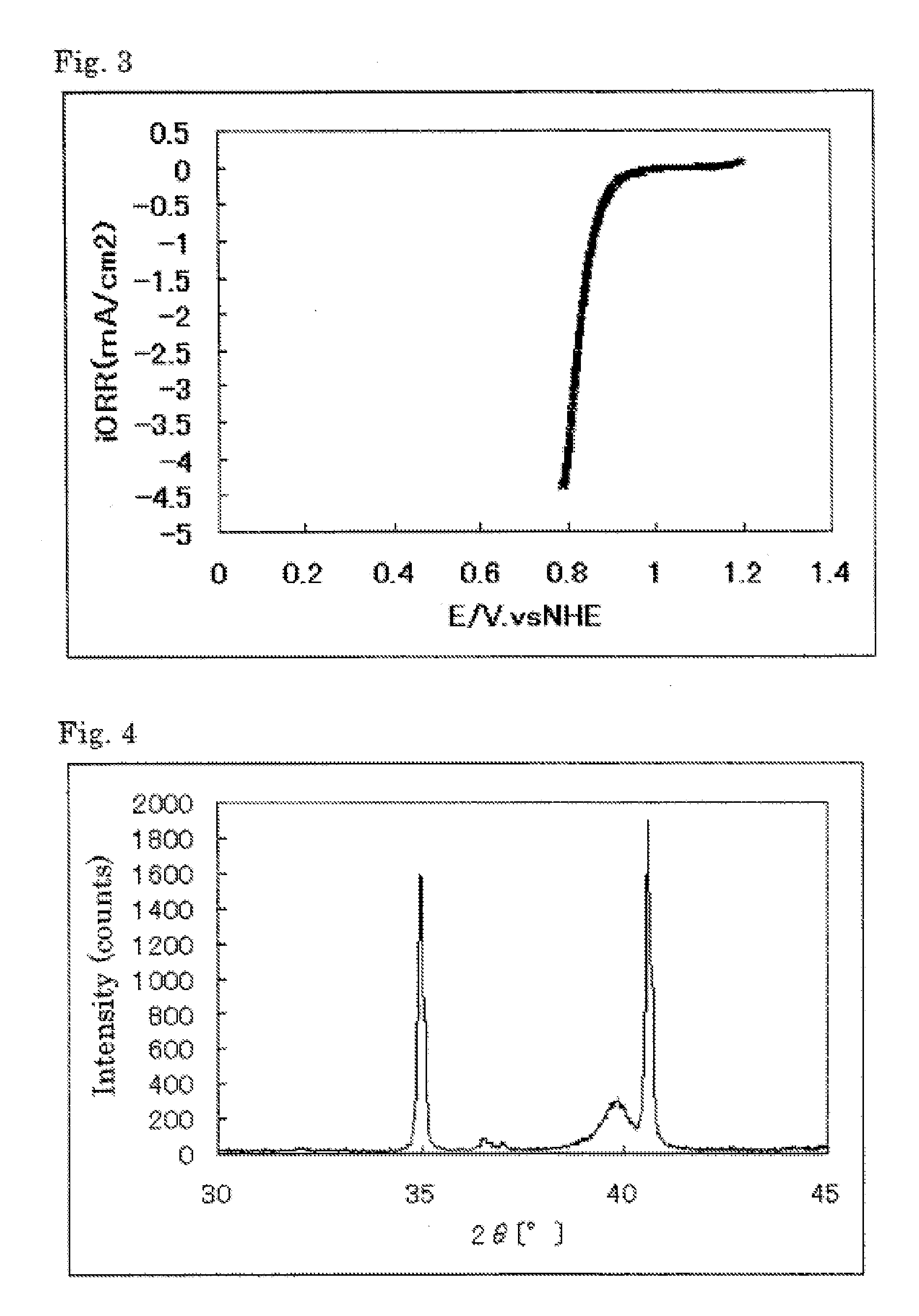
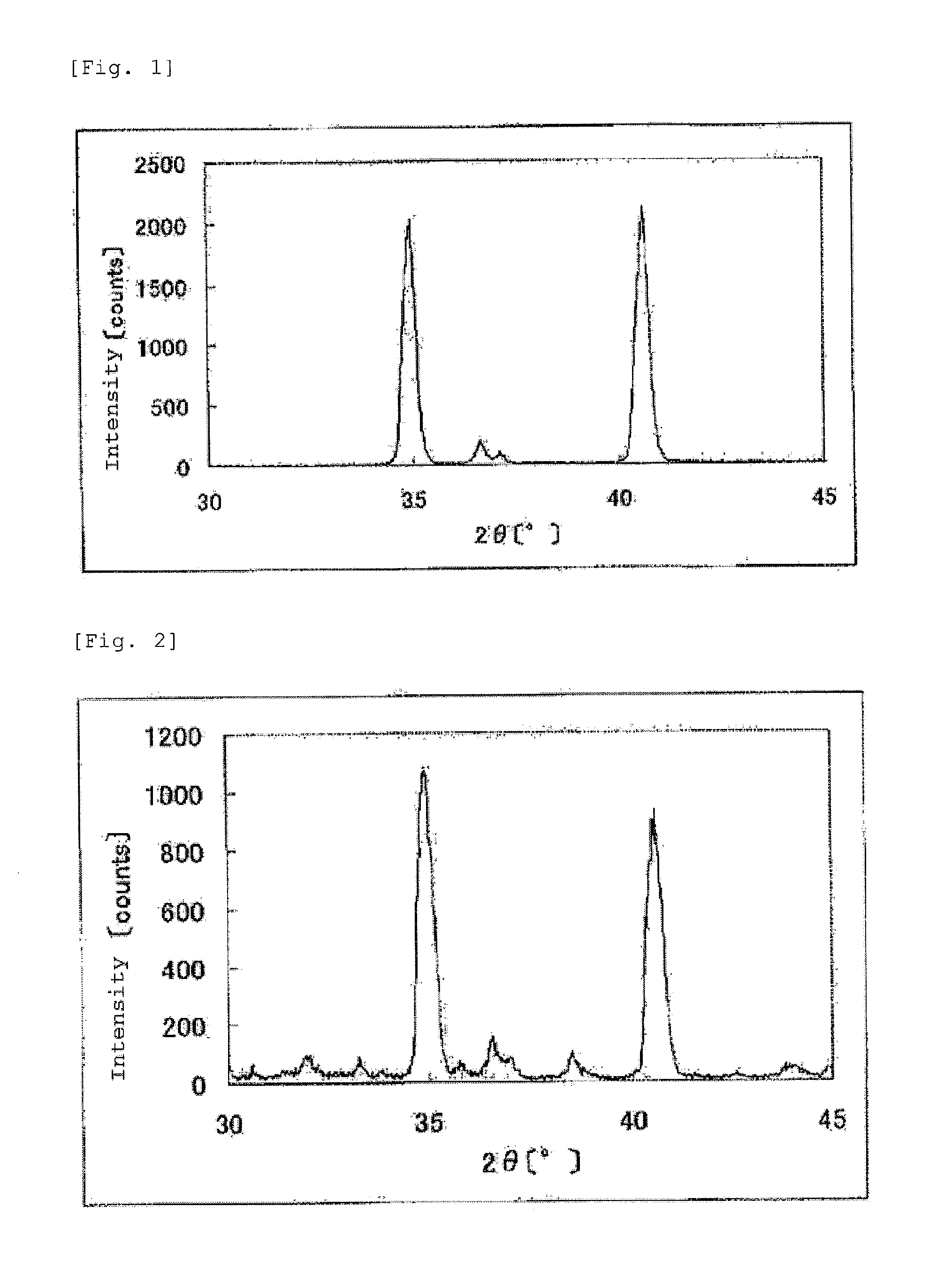
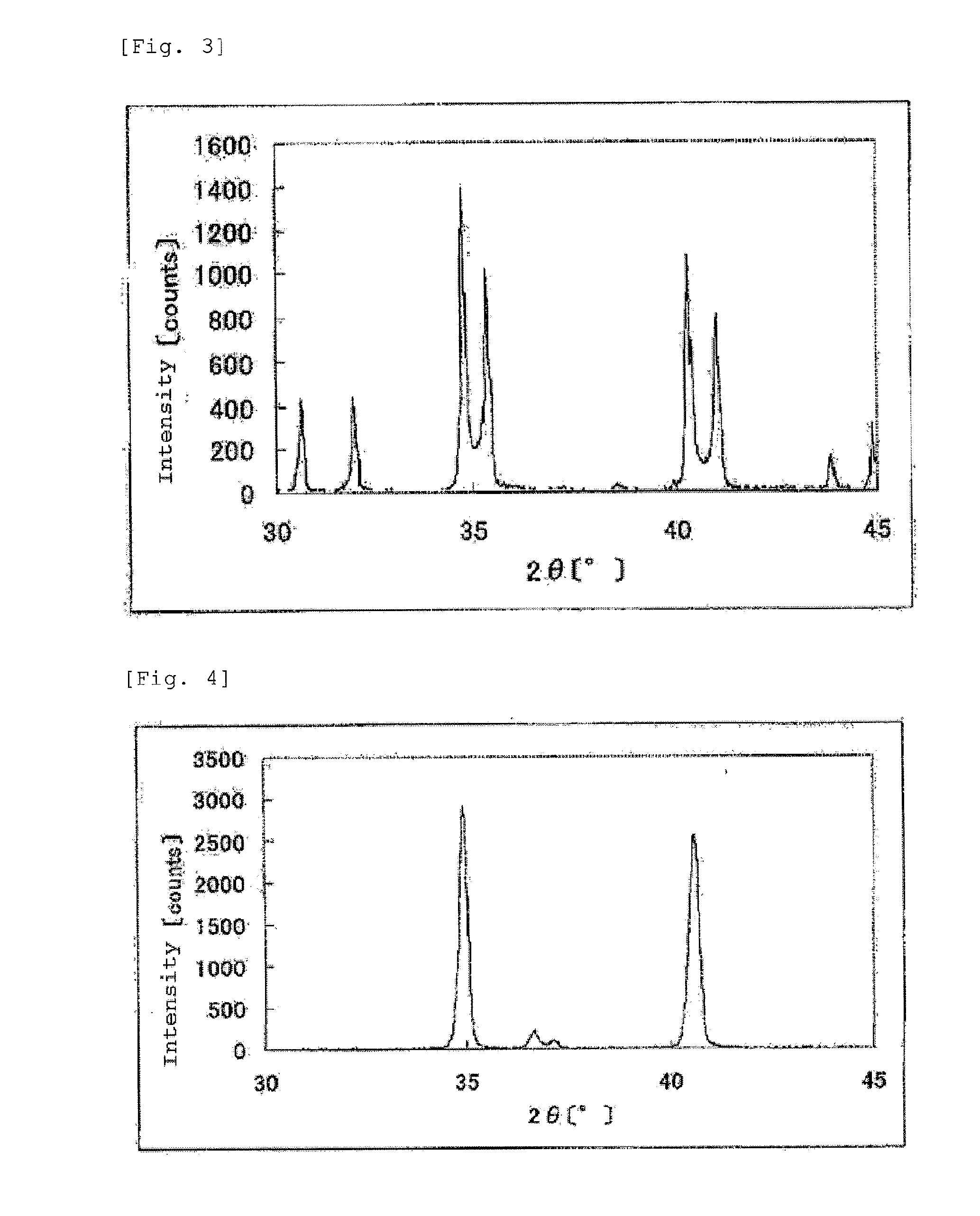
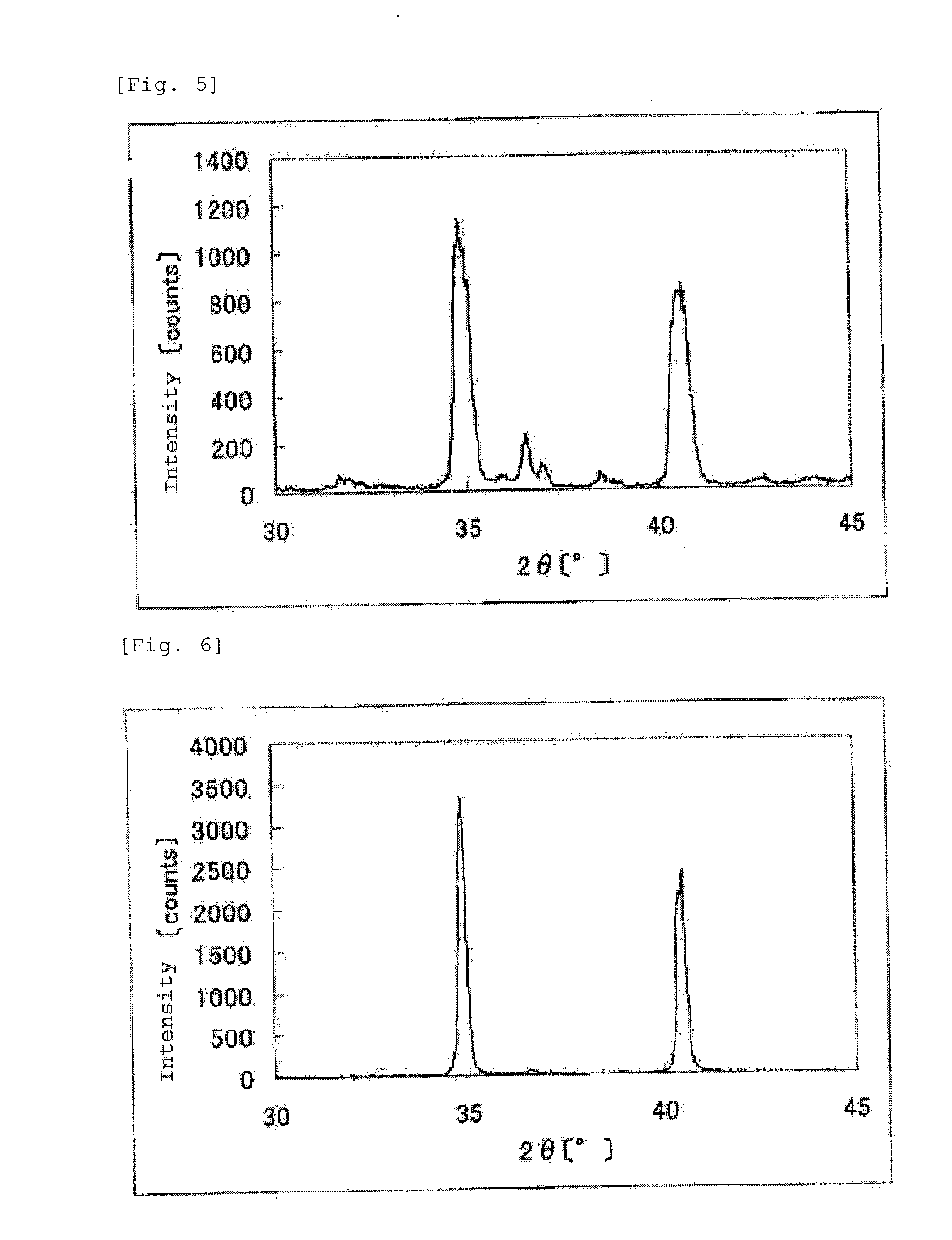
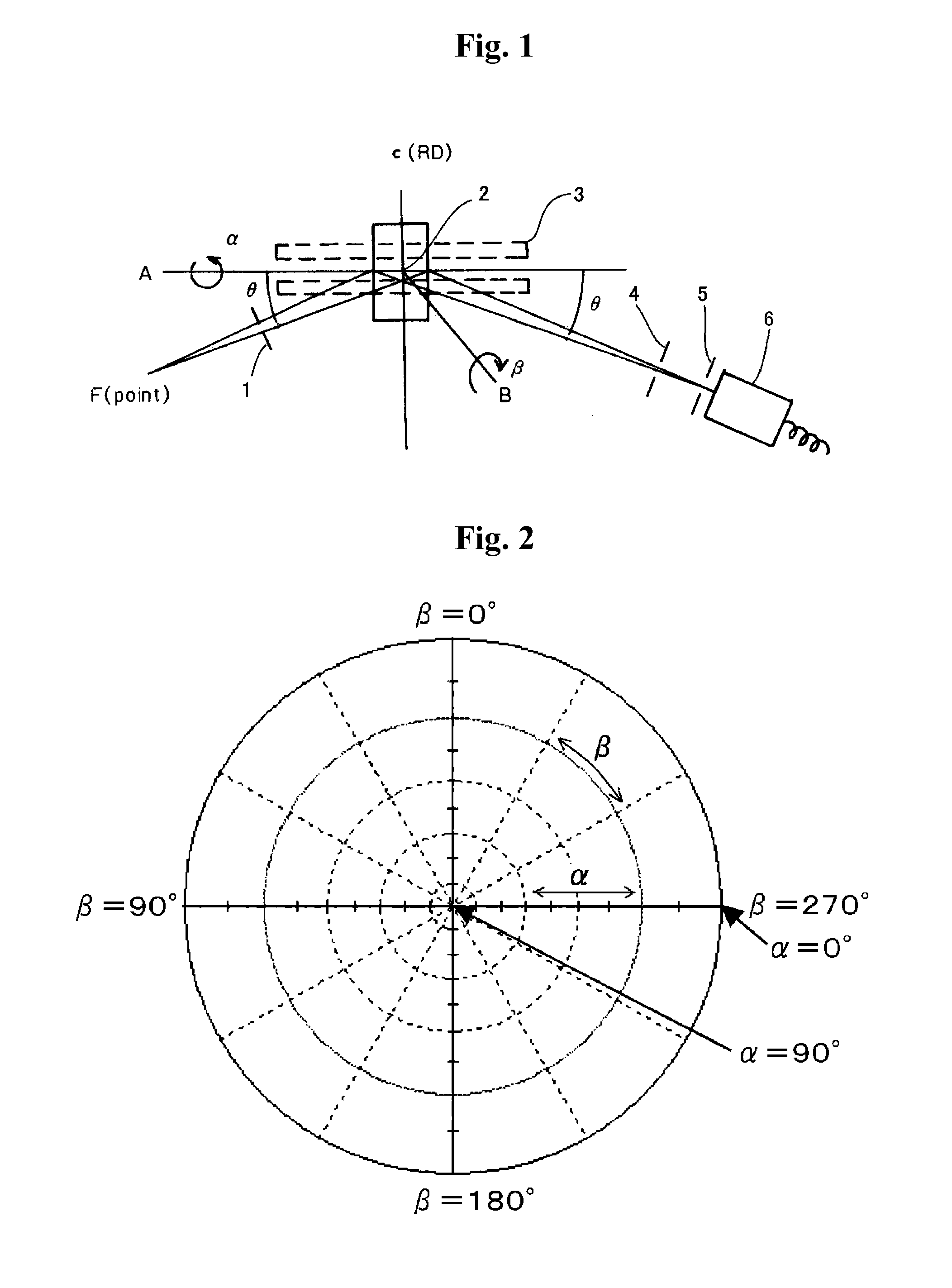
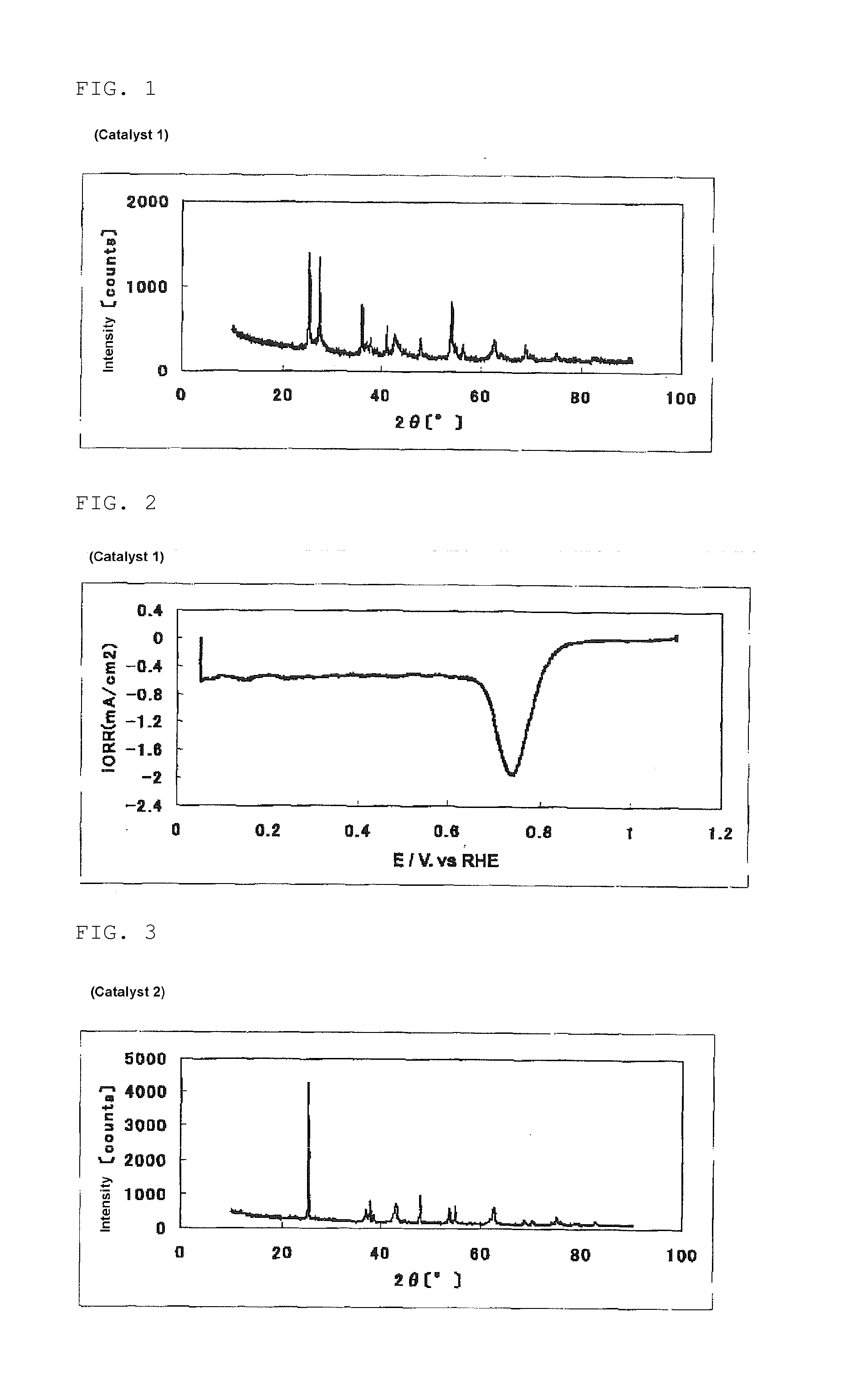
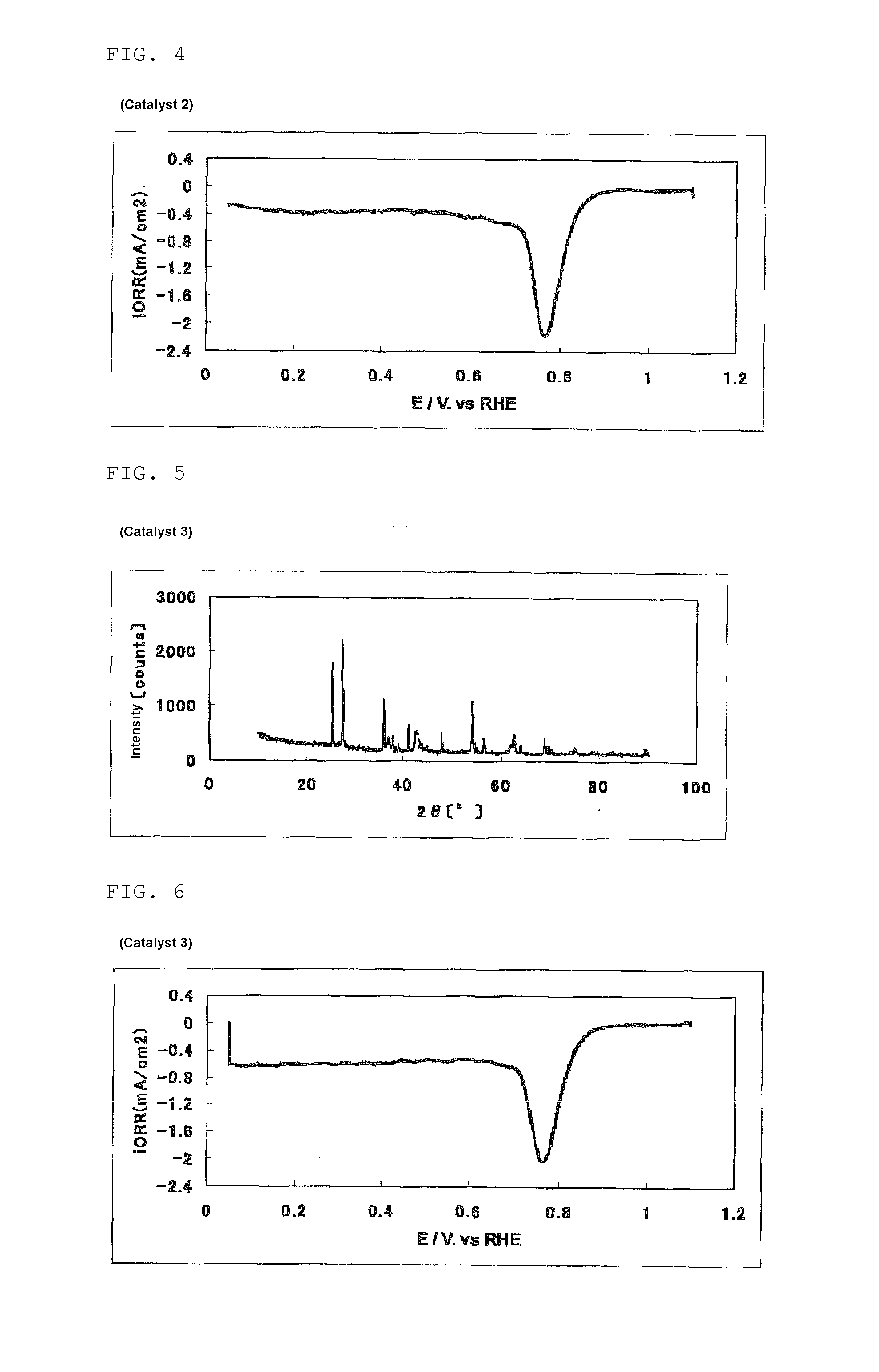
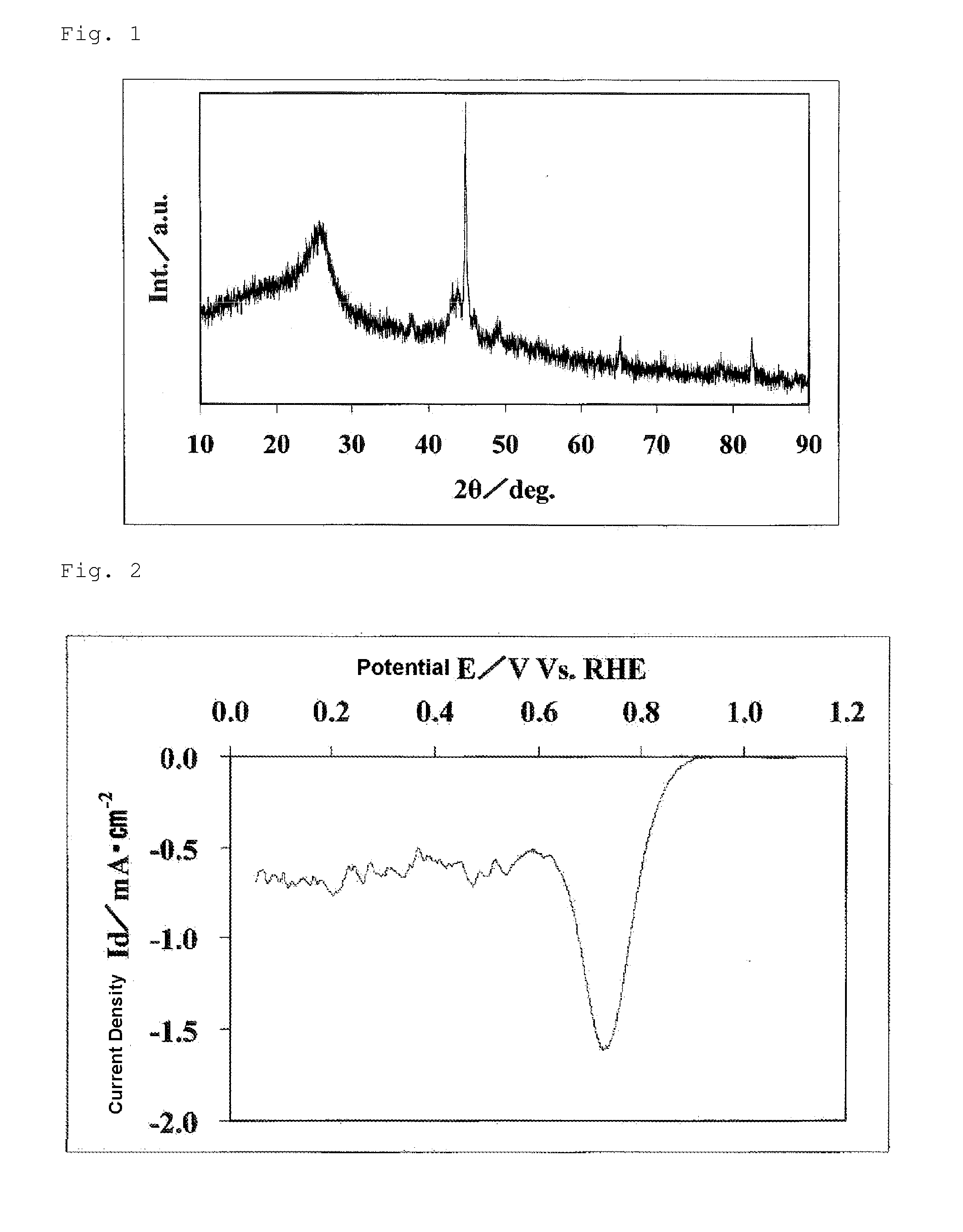
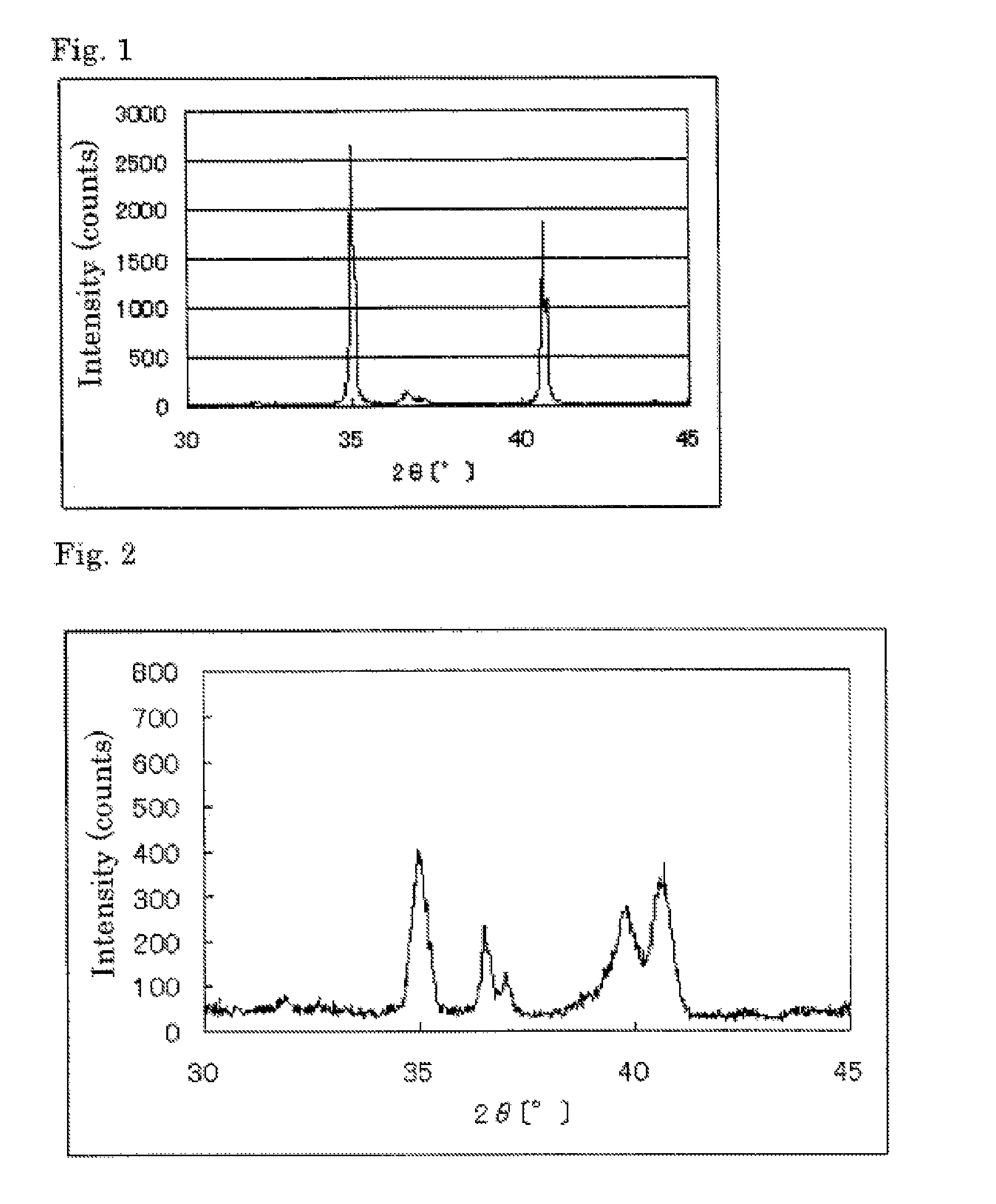
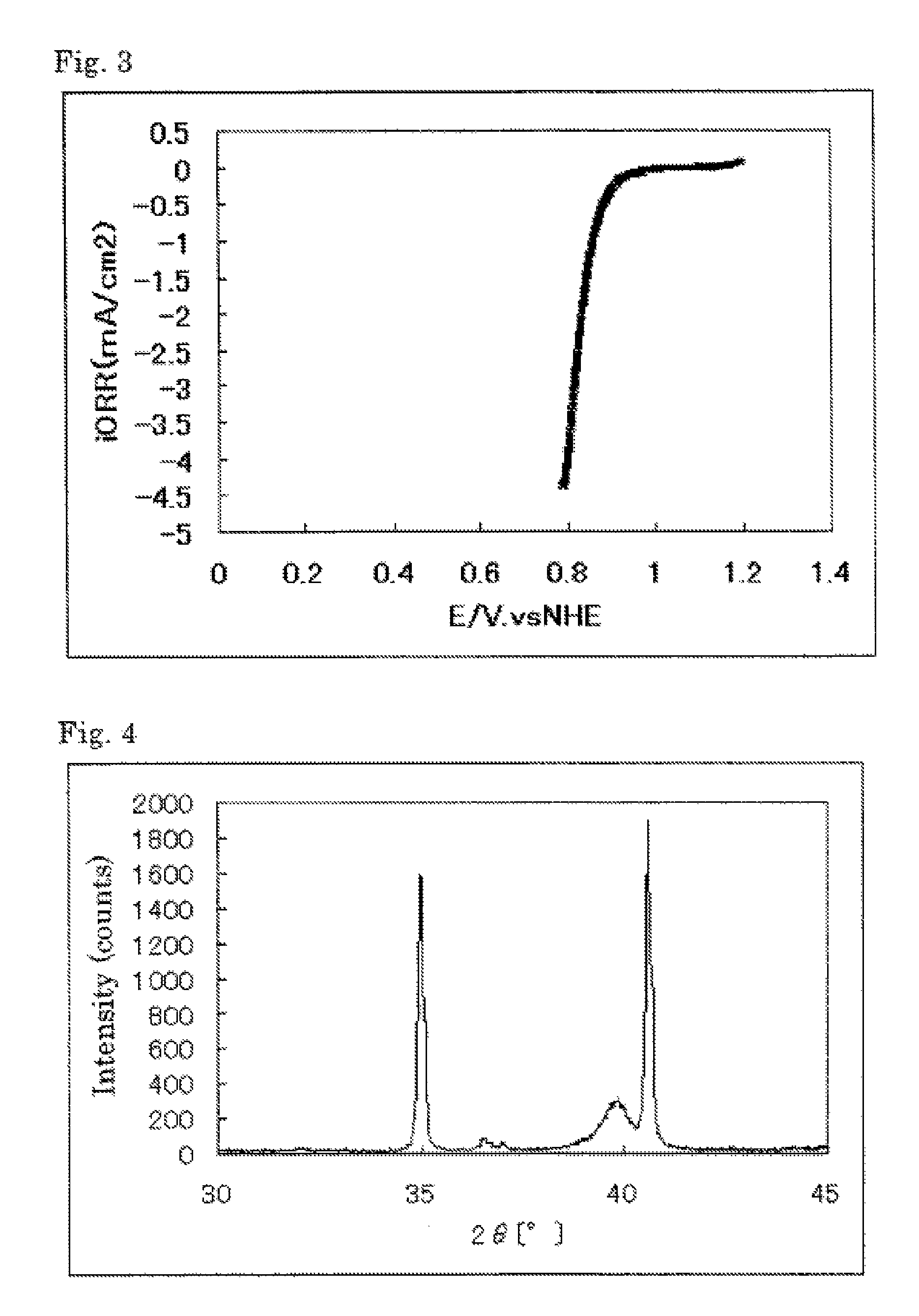
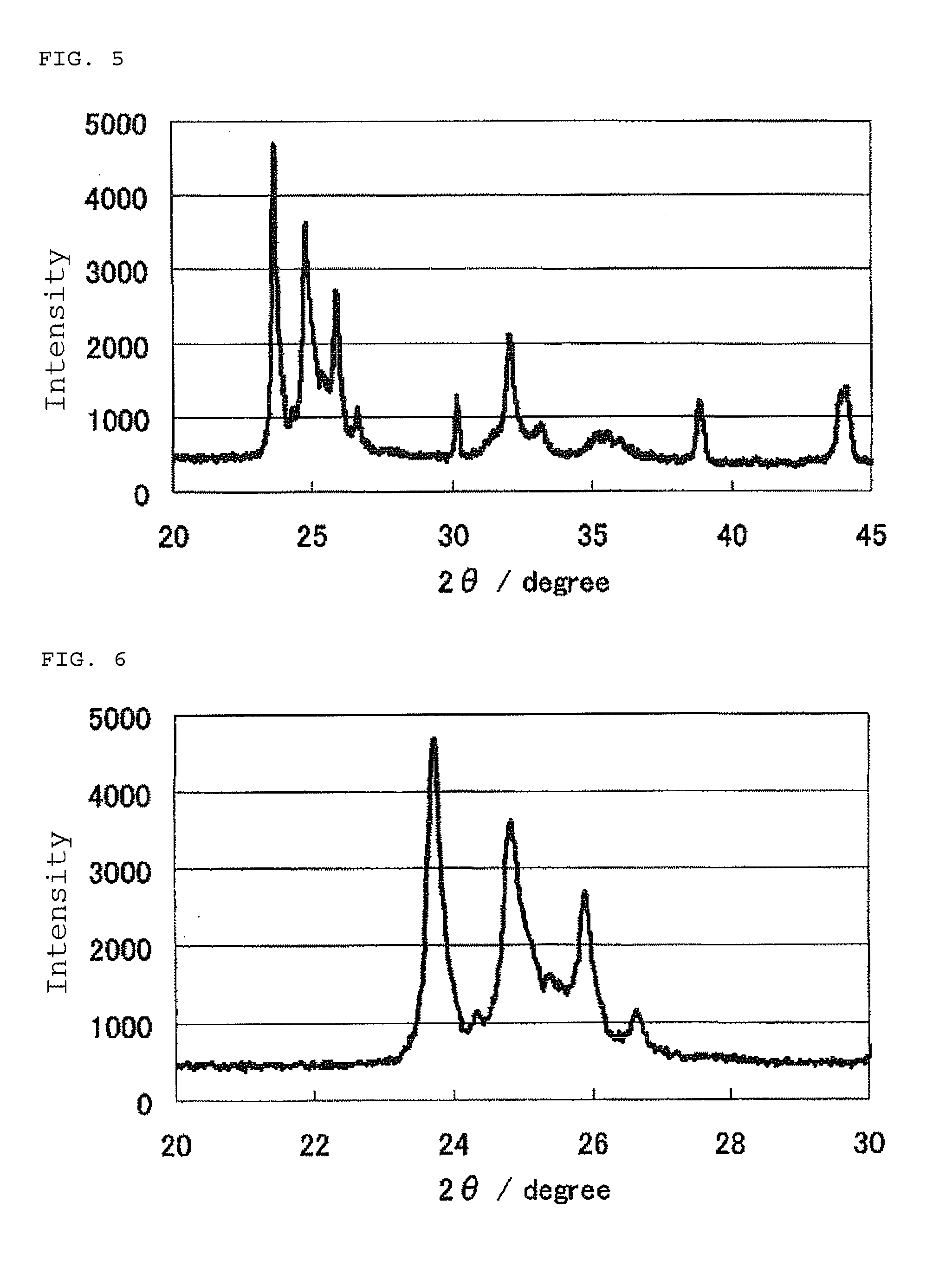
The invention provides catalysts which are not corroded in acidic electrolytes or at high potential and have excellent durability and high oxygen reducing ability.The catalysts include a niobium-containing oxycarbonitride having I2 / (I1+I2) of not less than 0.25 wherein I1 is the maximum X-ray diffraction intensity at diffraction angles 2θ of 25.45° to 25.65° and I2 is the maximum X-ray diffraction intensity at diffraction angles 2θ=2θ of 25.65° to 26.0° according to X-ray powder diffractometry (Cu—Kα radiation).
Owner:SHOWA DENKO KK
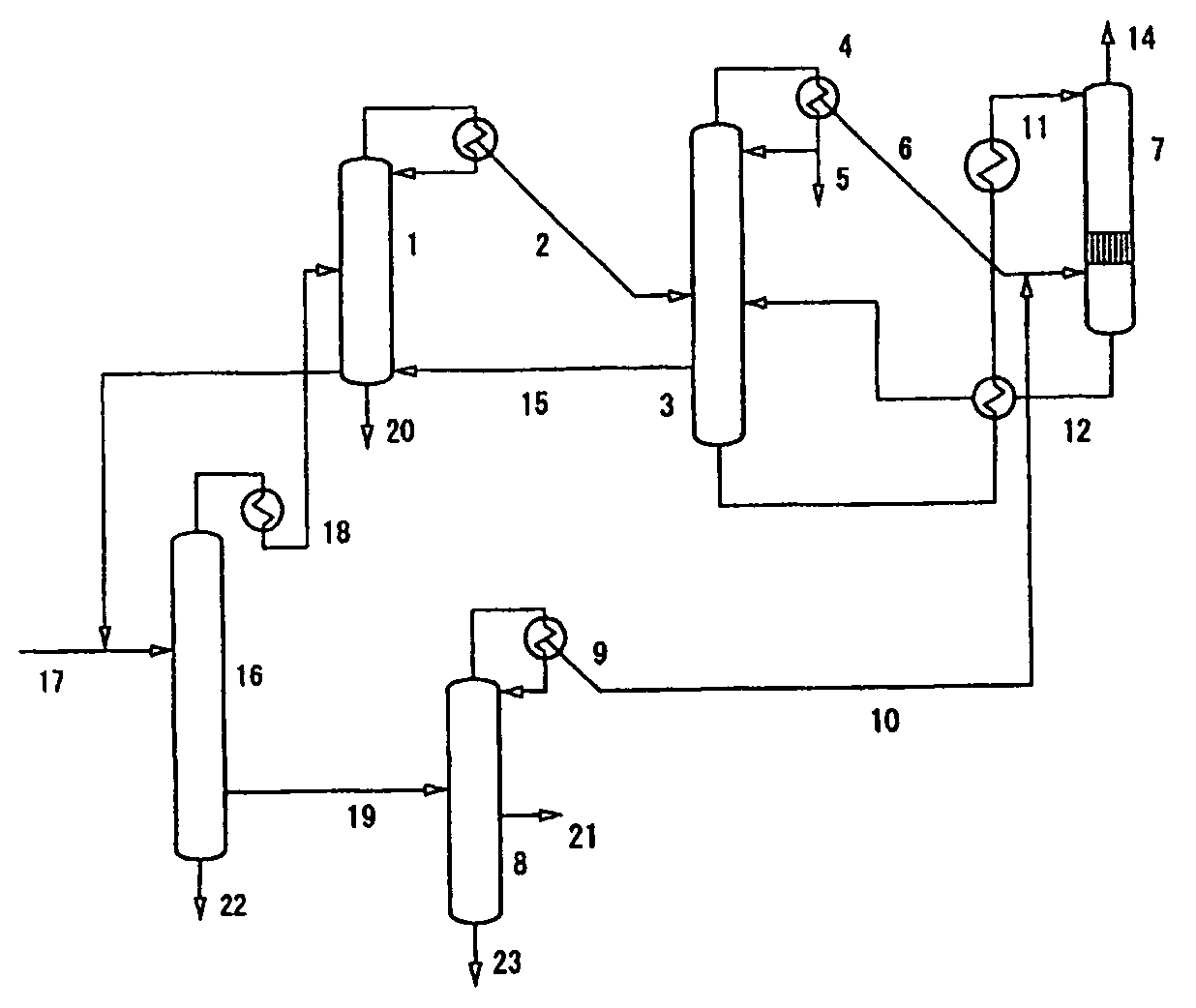
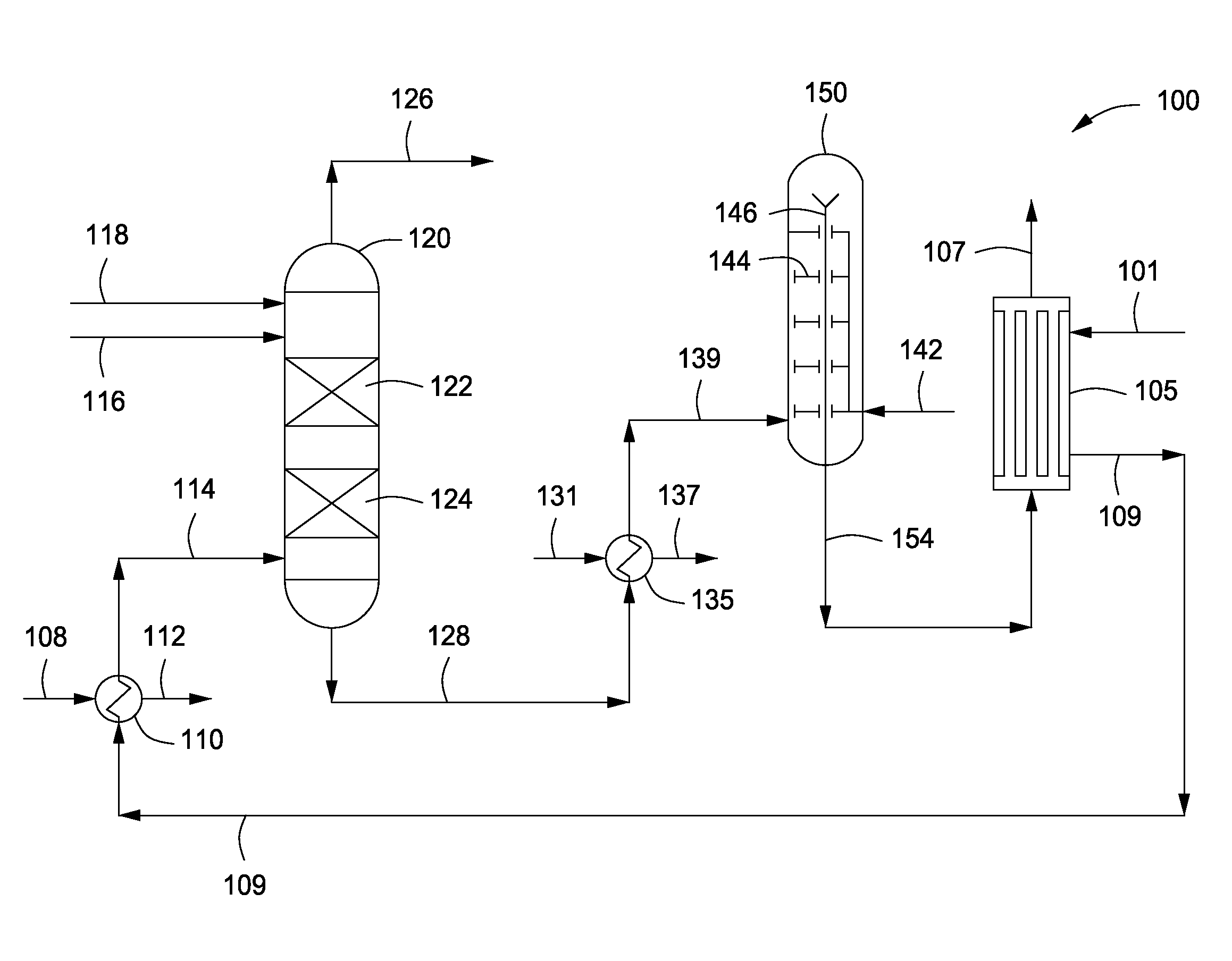
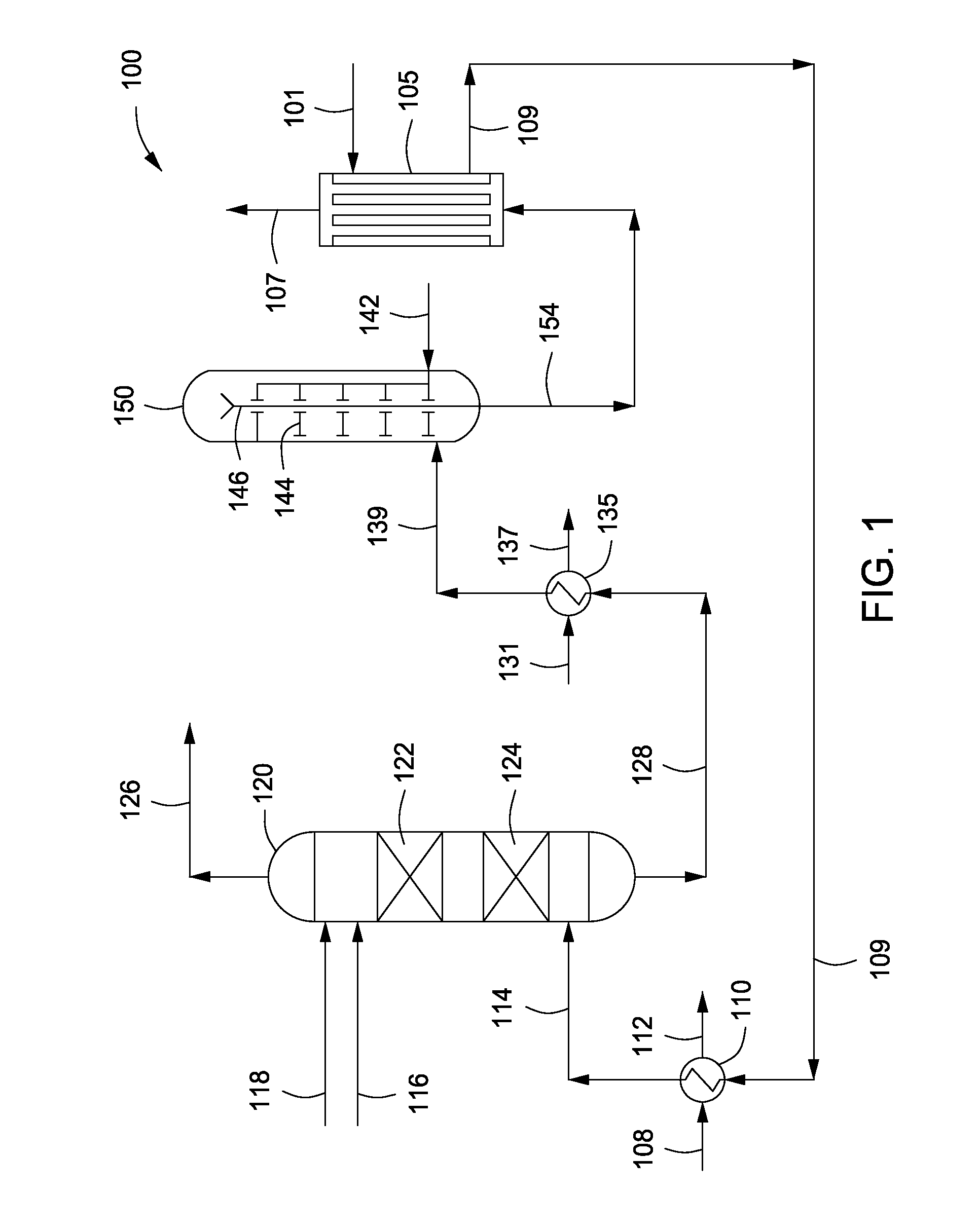
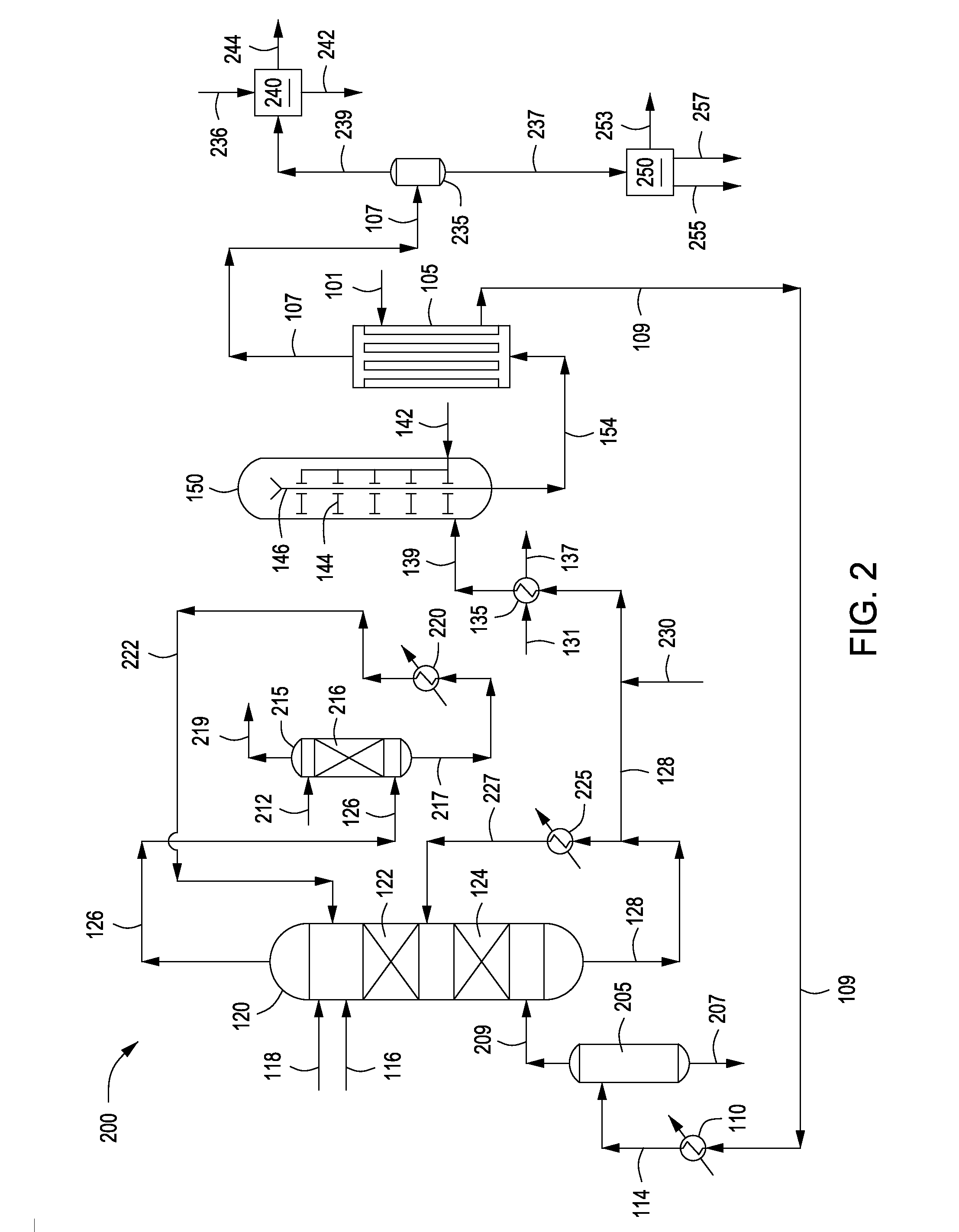
Process for Urea Production and Related Plant
ActiveUS20080219900A1Increase pressureSimple and cost-effectiveUrea derivatives preparationProductsDecompositionAmmonium carbamate
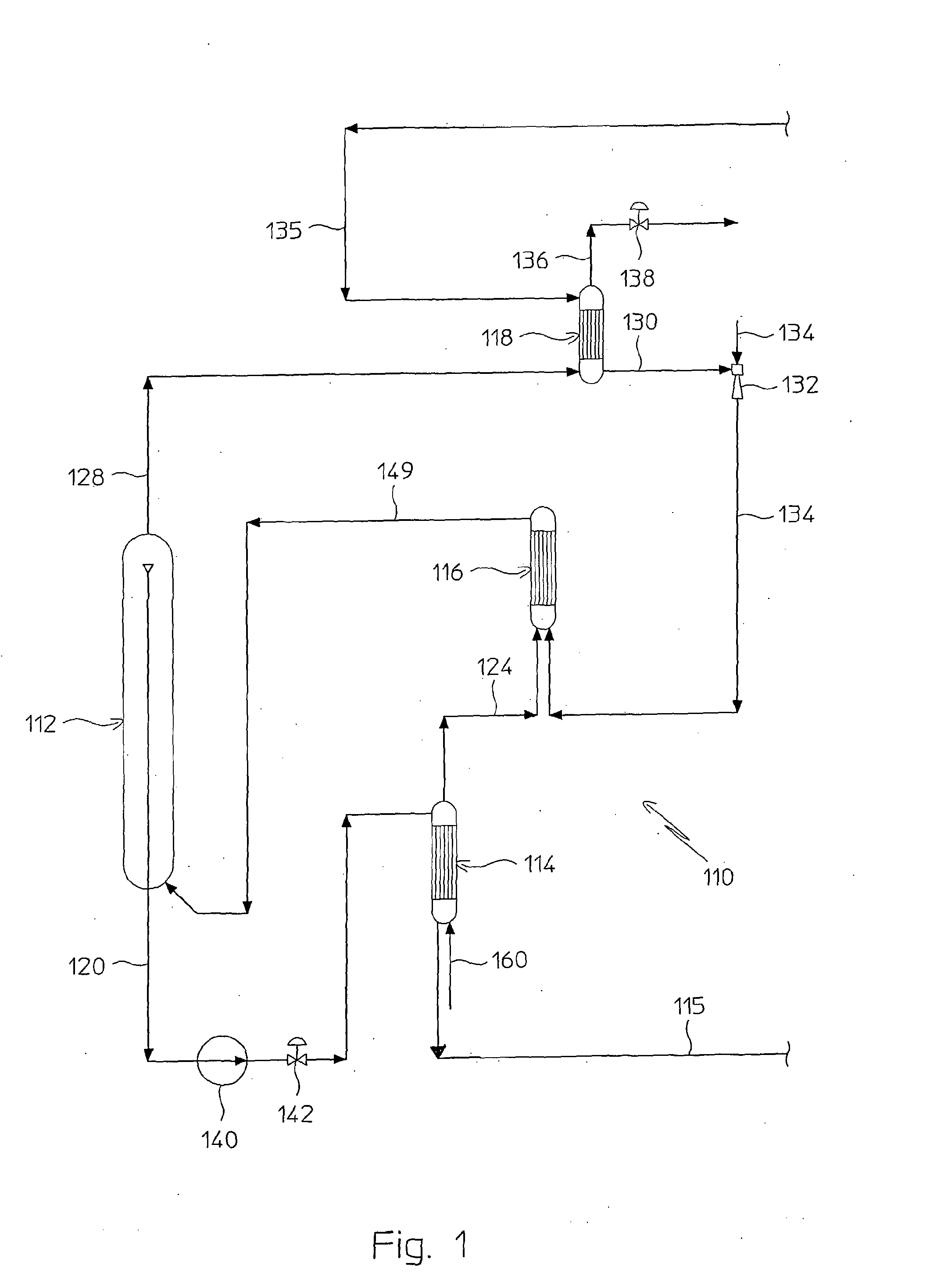
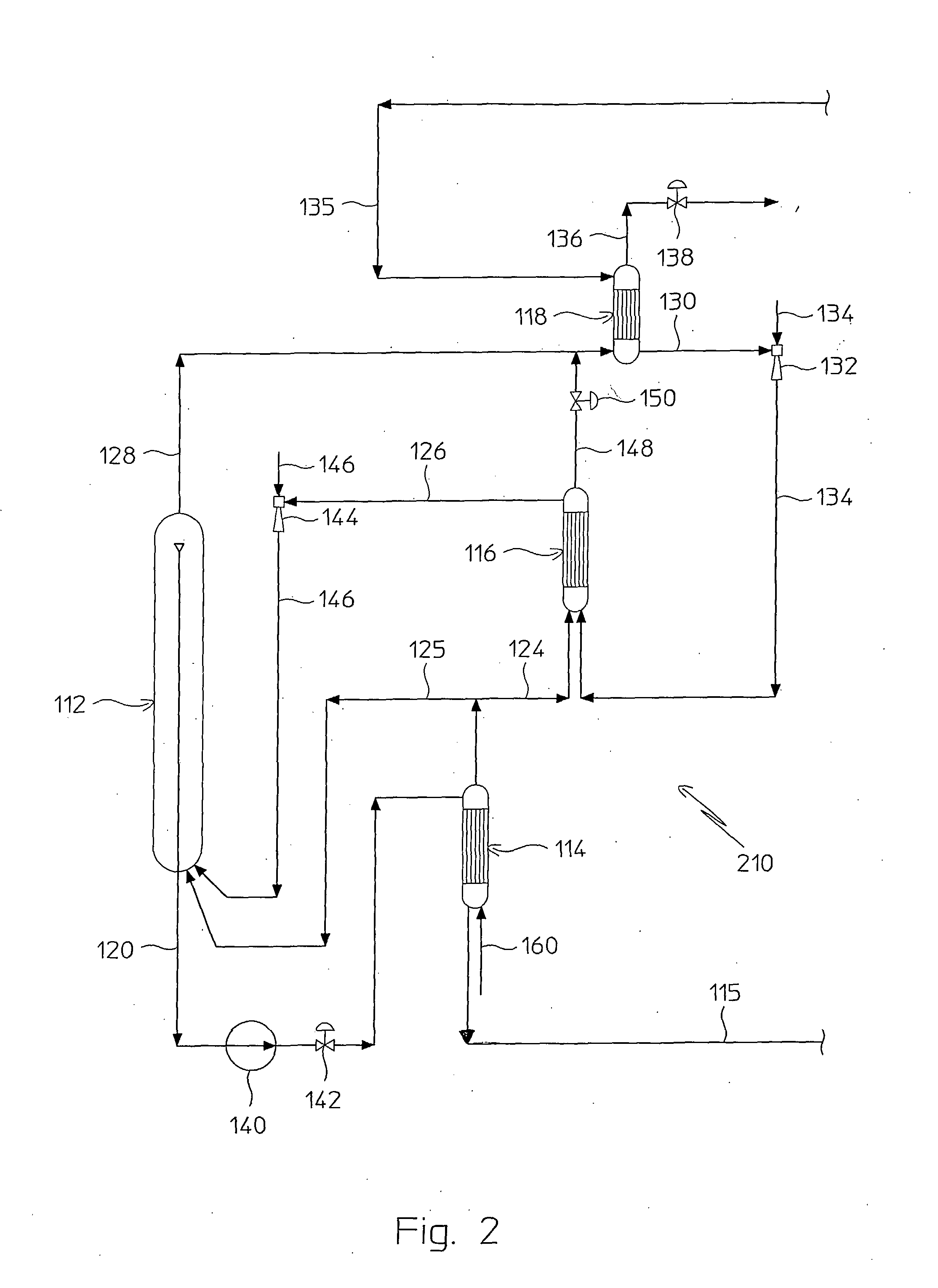
A process for urea production from ammonia and carbon dioxide, made to react at a predetermined high pressure in an appropriate synthesis reactor (112), from the reaction between NH3 and CO2 being obtained a reaction mixture comprising urea, ammonium carbamate and free ammonia in aqueous solution, from which a recovery of ammonium carbamate and ammonia is carried out with their subsequent recycle to the synthesis reactor (112), said recovery from the reaction mixture taking place through operative steps of decomposition of the ammonium carbamate into NH3 and CO2 and of their stripping and a subsequent operative step of their recondensation into ammonium carbamate that is recycled to the synthesis reactor, the said reaction mixture obtained from the reaction between ammonia and carbon dioxide being pumped to the operative steps of decomposition and stripping.
Owner:CASALE SA
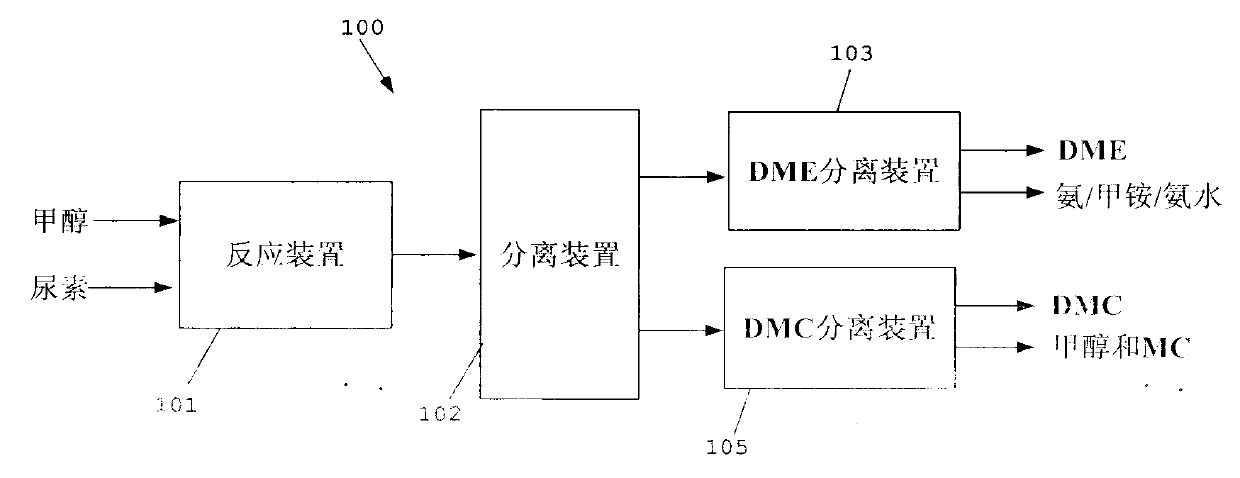
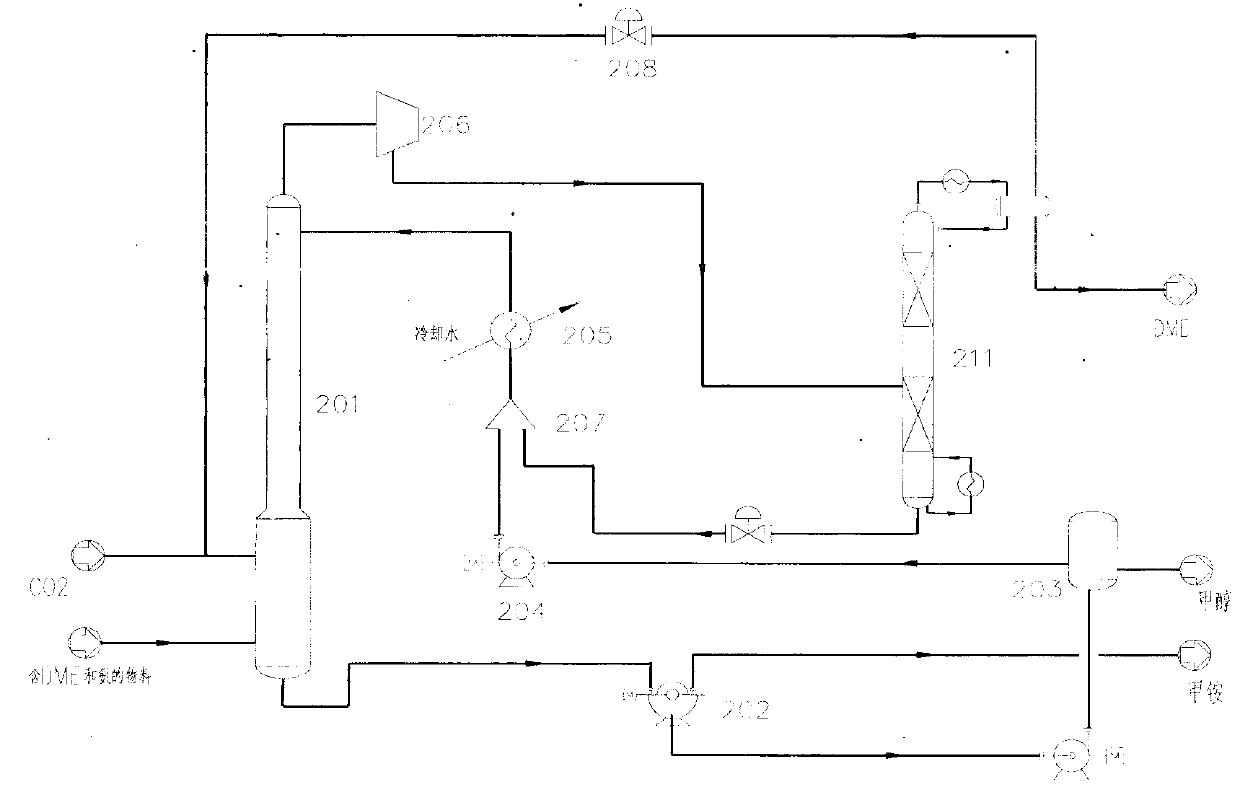
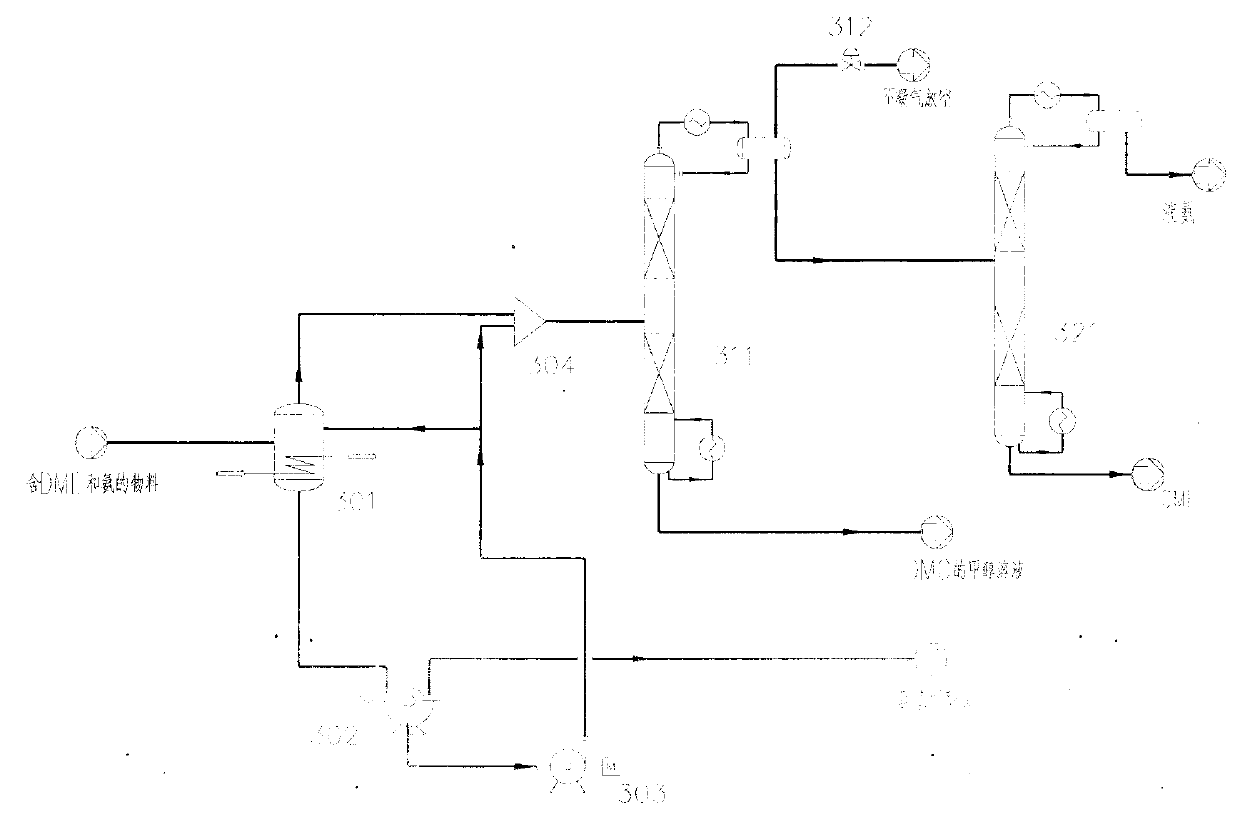
Method for separating dimethyl ether and recovering ammonia in co-production process for dimethyl carbonate and dimethyl ether via urea alcoholysis method
ActiveCN103288645AMeet supplyReduce manufacturing costEther separation/purificationOrganic compound preparationMethyl carbamateMethyl carbonate
The invention provides a method for separating dimethyl ether and recovering ammonia in a co-production process for dimethyl carbonate and dimethyl ether via a urea alcoholysis method. The method comprises the following steps of: adding methanol and urea in a reaction device, carrying out a reaction on methanol and urea to generate methyl carbamate and a by-product of ammonia, further carrying out a reaction on the generated methyl carbamate and methanol to generate dimethyl carbonate and the by-product of ammonia, and decomposing a part of dimethyl carbonate to generate dimethyl ether; separating out a material containing dimethyl ether and ammonia, and a material containing dimethyl carbonate from the output material of a reaction device; and separating out dimethyl ether from the material containing dimethyl ether and ammonia, converting ammonia to an ammonium carbamate solid, liquid ammonia or ammonia water, and recovering.
Owner:YASHENTECH CORP
Method for Producing Chlorosulfonyl Isocyanate
ActiveUS20070286789A1Easy to controlSulfonic acid amide preparationCyanic/isocyanic acidSulfur trioxideChlorosulfonyl isocyanate
A method for producing chlorosulfonyl isocyanate by reaction of sulfur trioxide with cyanogen chloride, wherein chlorosulfonyl isocyanate or a solution including chlorosulfonyl isocyanate is used as a reaction solvent, sulfur trioxide and cyanogen chloride which are respectively diluted with the chlorosulfonyl isocyanate or the solution including chlorosulfonyl isocyanate are added at the same time to a reaction system in an almost equimolar amount under reflux. By the production method of present invention, chlorosulfonyl isocyanate can be produced from sulfur trioxide and cyanogen chloride in which the yield of the chlorosulfonyl isocyanate is high, the method has excellent operability, number of equipments is reduced, and time for controlling the temperature is saved.
Owner:NIPPON SODA CO LTD
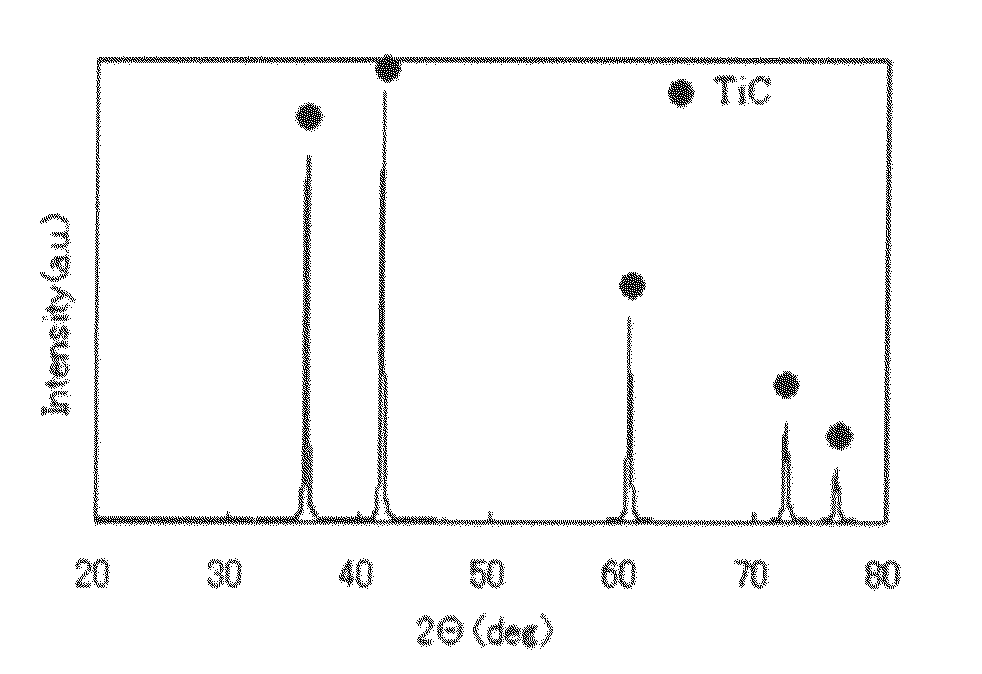
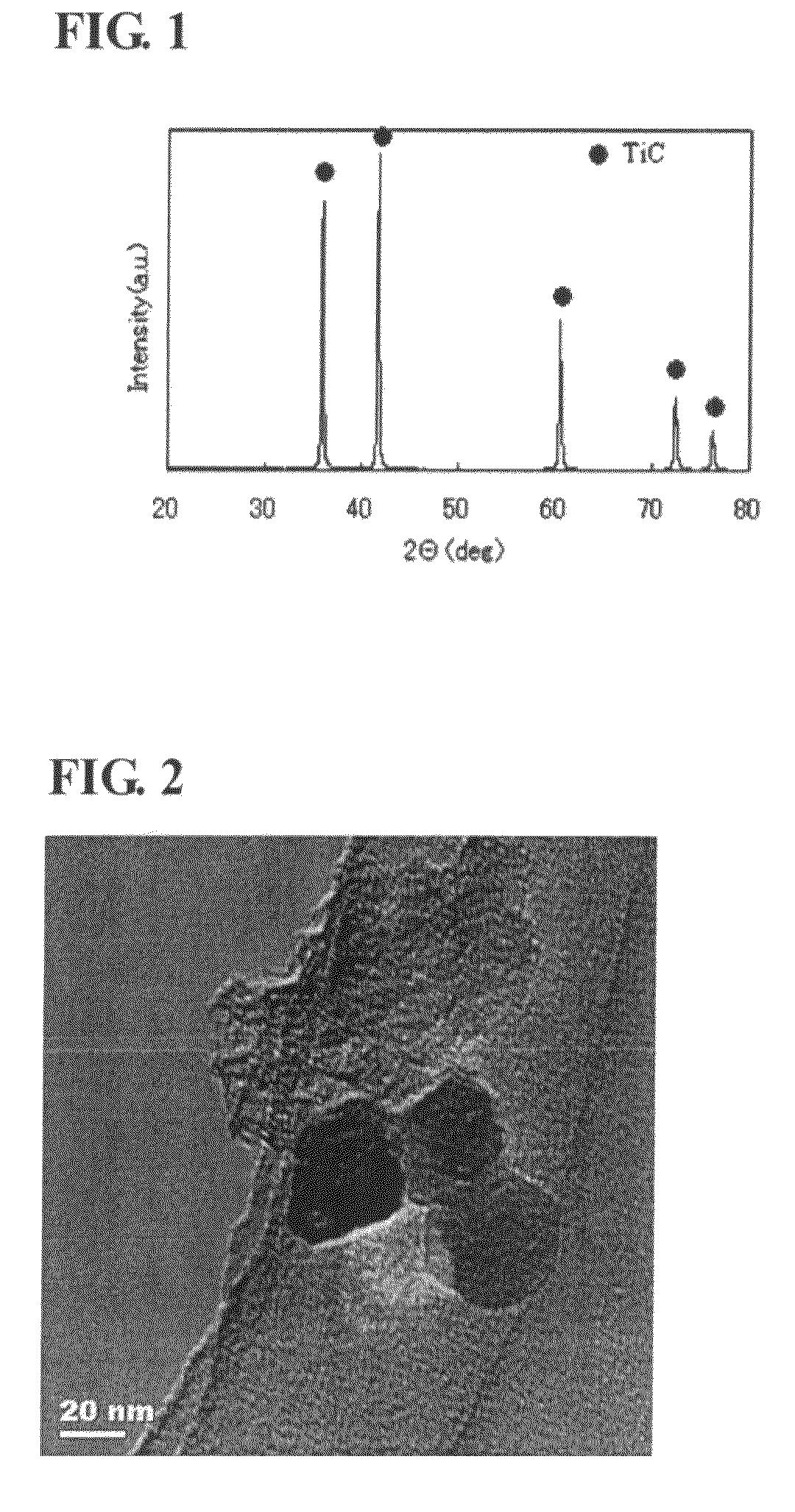
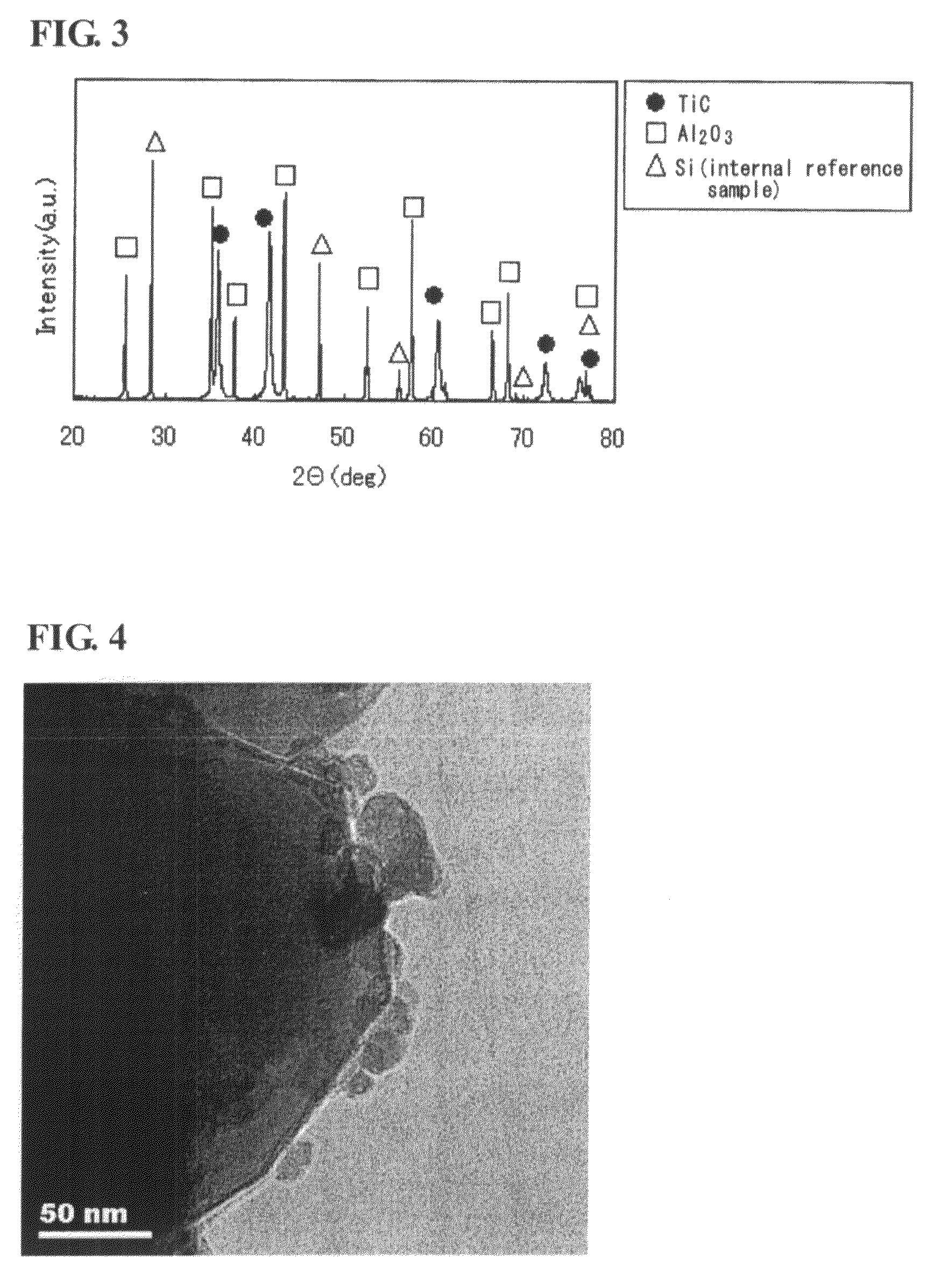
Titanium carbide powder and titanium carbide-ceramics composite powder and method for production thereof, and sintered compact from the titanium carbide powder and sintered compact from the titanium carbide/ceramics composite powders and method for production thereof
InactiveUS7915187B2Improve batch productivityReduce the amount requiredMaterial nanotechnologyTitanium carbideCeramic compositeGranularity
Owner:FUKUOKA PREFECTURAL GOVERNMENT +1
Continuous method and apparatus for functionalizing carbon nanotube
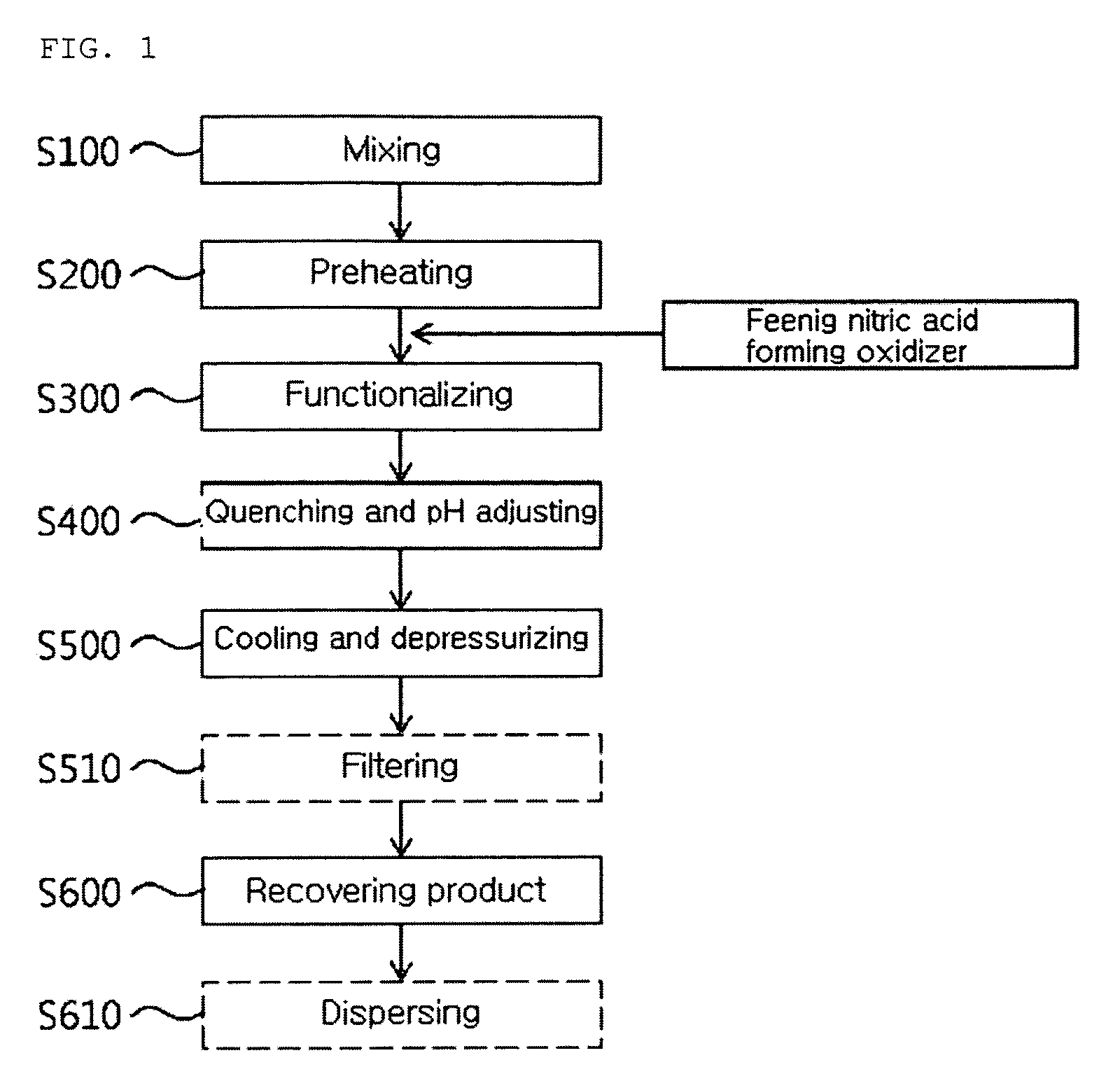
ActiveUS20090269267A1Good effectHigh viscosityMaterial nanotechnologyNanostructure manufactureArylNitro compound
The present invention relates to a continuous method and apparatus for functionalizing a carbon nanotube, and more specifically, to a continuous method and apparatus for functionalizing a carbon nanotube including preparing a functionalized product by functionalizing a carbon nanotube solution including nitro compound according to the following Chemical Formula 1 and carbon nanotube mixture including an oxidizer for forming nitric acid under subcritical water or supercritical water condition of 50 to 400 atm and a continuous method and apparatus for functionalizing a carbon nanotube under subcritical water or supercritical water condition using nitro compound without using strong acids or strong bases.R—(NOx)y [Chemical Formula 1]wherein Chemical Formula 1, R is alkyl group of C1 to C7 or aryl group of C6 to C20 and x and y are integers of 1 to 3 independently.
Owner:HANWHA CHEMICAL CORPORATION
Ammonium recovery methods
ActiveUS8580219B2Effective recoveryImprove solubilitySludge treatmentMicroorganismsLiquid productSyngas
The methods are utilized to recover ammonium from waste water using CO2 acidified absorption water. The process is particularly suited for utilization of cellular matter and a CO2 rich tail gas from a syngas fermentation process and derives significant benefit from the recovery of ammonium bicarbonate and ammonium carbonate. Ammonia and ammonium are recovered from the treatment of the syngas as an ammonium rich solution, at least a portion of which is recycled to the fermentation zone to aid in the production of liquid products. A carbon dioxide rich gas produced by fermentation is used to capture the ammonia and ammonium, forming the ammonium rich solution.
Owner:SYNATA BIO INC
Catalyst, production process therefor and use thereof
InactiveUS8637206B2Raise the potentialImprove performanceCatalyst protectionFinal product manufactureCrystallographyNiobium
The invention provides catalysts which are not corroded in acidic electrolytes or at high potential and have excellent durability and high oxygen reducing ability. The catalysts include a niobium-containing oxycarbonitride having I2 / (I1+I2) of not less than 0.25 wherein I1 is the maximum X-ray diffraction intensity at diffraction angles 2θ of 25.45 to 25.65 degrees and I2 is the maximum X-ray diffraction intensity at diffraction angles 2θ=2θ of 25.65 to 26.0 degrees according to X-ray powder diffractometry (Cu—Kα radiation).
Owner:SHOWA DENKO KK
Systems and methods for forming a solution of ammonium carbamate
A method for forming a solution of ammonium carbamate is provided herein. The method includes, but is not limited to, providing a reactor comprising a solution of ammonia. The method further includes, but is not limited to, feeding carbon dioxide through the solution of ammonia to form a mixture. The method further includes, but is not limited to, combining a solution of sodium hydroxide and the mixture to form the ammonium carbamate.
Owner:SOLENIS TECH CAYMAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com