Polymerization method for catalyzing olefin by nickel-diimine catalytic system
A catalytic system, diimine technology, which is applied in the field of nickel diimine catalytic system to catalyze the polymerization of olefins, can solve the problems of low toxicity, high toxicity, and low boiling point, and achieve the effect of easy recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
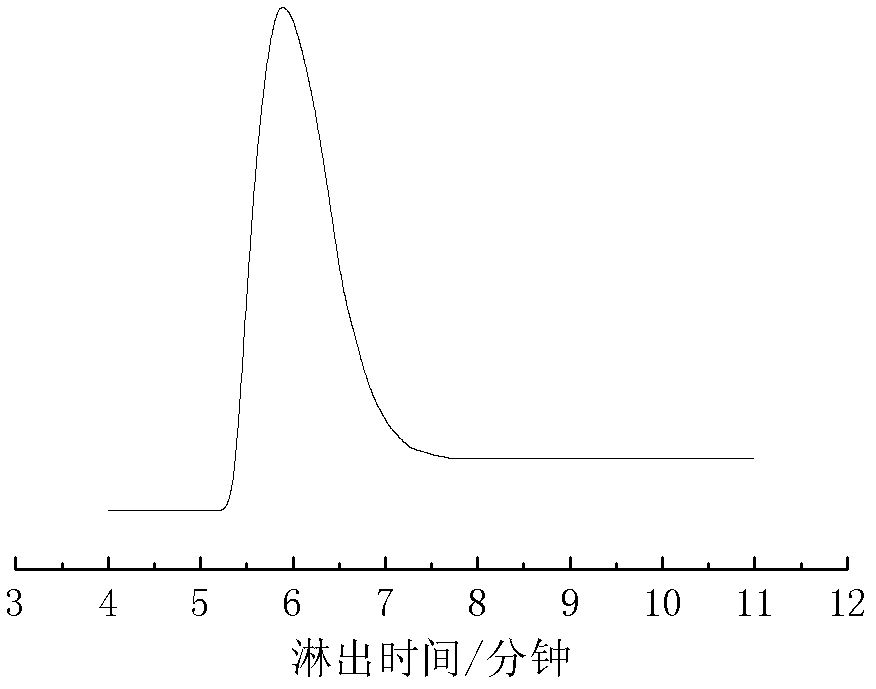
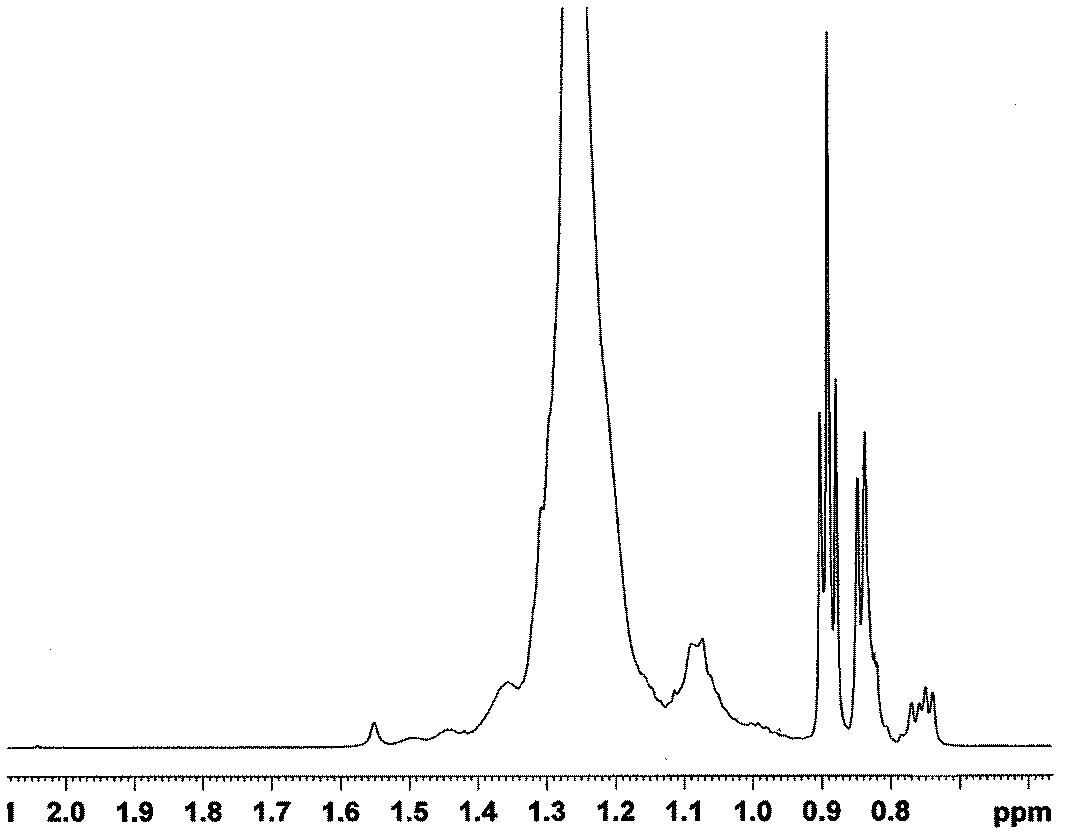
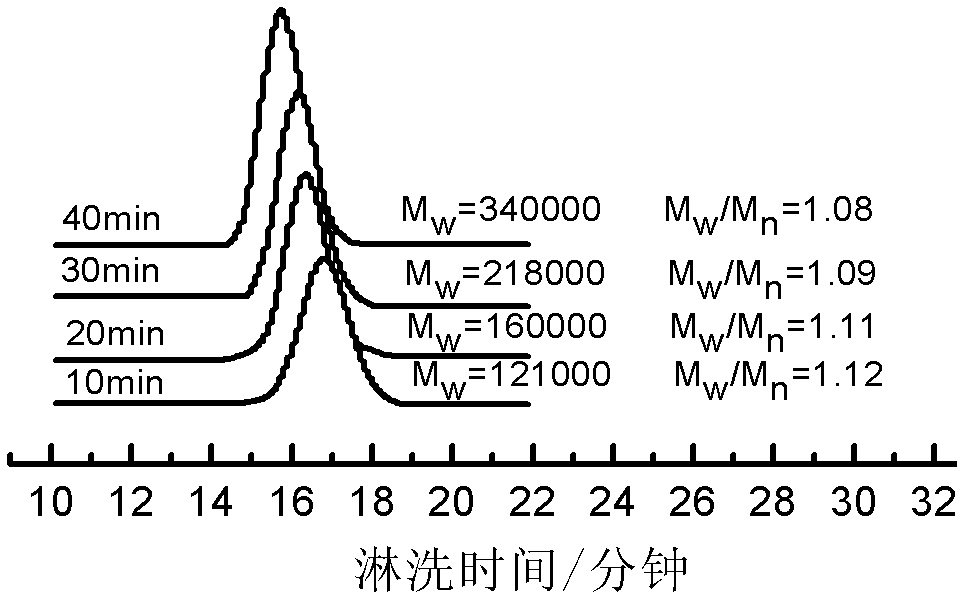
[0021] After washing the 2L reactor with n-hexane, replace it with ethylene three times, add 1000mL of n-hexane, catalyst [(2,6-i-PrPh) 2 DAB An]NiBr 2 0.012g (0.017mmol), seal the reaction kettle under ethylene atmosphere, inject 10mL (17mmol) methylalumoxane into the reaction kettle with a dry and nitrogen-washed syringe through the branch hose, and finally feed ethylene, keep 30 atmospheric pressure. The reaction stopped after stirring for 30 minutes at 60° C., and the reaction system was black and viscous. The reaction was terminated with dilute hydrochloric acid-methanol solution. The obtained polymer was dissolved in tetrahydrofuran and precipitated with methanol, and this was repeated three times. Finally, the sample was dried in a vacuum oven at room temperature for 24 hours to obtain 20 grams of a white solid polymer with certain elasticity, which was an amorphous polymer. Its glass Transition temperature Tg=-28°C. And the catalytic efficiency of this catalytic s...
Embodiment 2
[0023] After washing the 2L reactor with n-hexane, replace it with propylene three times, and add 1000mL of n-hexane, catalyst [(2,6-i-PrPh) 2 DAB An]NiBr 2 0.012g (0.017mmol), seal the reaction kettle under the atmosphere of propylene, inject 10mL (17mmol) methylalumoxane into the reaction kettle with a dry and nitrogen-washed syringe through the branch hose, and finally pass into propylene, keep 3 atmospheric pressure. The reaction stopped after stirring for 30 minutes at 60° C., and the reaction system was black and viscous. The reaction was terminated with dilute hydrochloric acid-methanol solution. The obtained polymer was dissolved in tetrahydrofuran and precipitated with methanol, and this was repeated three times. Finally, the sample was dried in a vacuum oven at room temperature for 24 hours to obtain 16 grams of a white solid polymer with certain elasticity, which was an amorphous polymer. Its glass Transition temperature Tg=-20°C. And the catalytic efficiency o...
Embodiment 3
[0025] Nitrogen gas-vacuumize-gas lamp baking bottle-nitrogen gas, repeat this three times, and the reaction bottle is cooled to normal temperature under the protection of nitrogen gas. Add 45 mL of n-hexane, 2.5 mL of 1-hexene and catalyst [(2,6-i-PrPh) 2 DAB An]NiBr 2 0.0012g (0.0017mmol), seal the reaction bottle and ventilate nitrogen to maintain a positive pressure in the reaction bottle, and finally inject 1mL (1.7mmol) methylalumoxane into the reaction bottle through a branch hose with a dry and nitrogen-washed syringe. The reaction stopped after stirring for 2 hours at 60° C., and the reaction system was black and viscous. The reaction was terminated with dilute hydrochloric acid-methanol solution. The obtained polymer was dissolved in tetrahydrofuran and precipitated with methanol, and this was repeated three times. Finally, the sample was dried in a vacuum oven at room temperature for 24 hours to obtain 2.1 grams of a white solid polymer with certain elasticity, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com