6-aliphatic hydrocarbon amido hexyl hydroximic acid collecting agent and preparation and application methods thereof
A technology of base hexyl hydroxamic acid and base hexyl hydroxamic acid salt, which is applied to 6-aliphatic hydrocarbon amido hexyl hydroxamic acid collector and its preparation and application fields, can solve the problem of low product yield, amide bond cleavage, Acyl chloride is highly toxic, and the flotation recovery rate is improved, the chelation capacity is strong, and the flotation separation effect is high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the preparation of 6-nordecyl amidohexyl hydroxamic acid
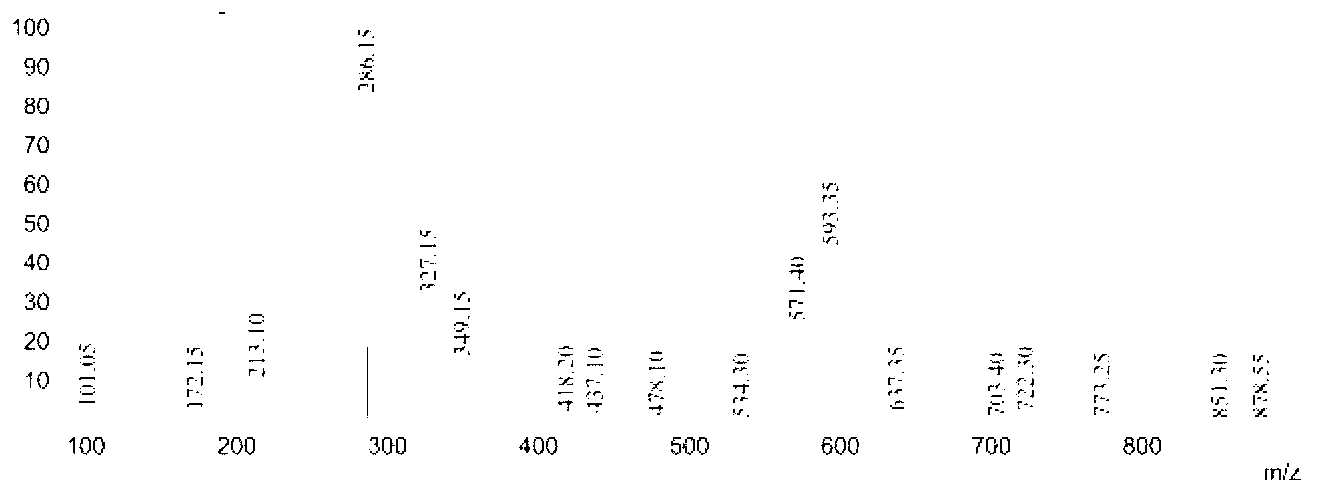
[0045] Add 11.32 parts of caprolactam with a purity of 99% and 6.95 parts of hydroxylamine hydrochloride with a purity of 99% into the reactor, add 34.64 parts of toluene as a solvent, heat to 105°C under stirring, and react for 3 hours, distill out the solvent for recovery; then Add 17.26 parts of n-decanoic acid into the above reactor, heat to 120° C. under stirring, and react for 1 to 4 hours to obtain the desired 6-n-decylamidohexyl hydroxamic acid product. Analysis showed that the purity of 6-n-decylamidohexyl hydroxamic acid was 90.3%, and the yield was 92.4%. Product Mr: 300.24, detected by mass spectrometry MS: 286.15 [M-15] (see figure 1 ), which is the peak of demethylation. NMR analysis and infrared spectrum analysis spectra are shown in Figure 5 with Figure 8 , see Table 1 and Table 2 for the data.
Embodiment 2
[0046] Embodiment 2: the preparation of 6-n-octylaminohexyl hydroxamic acid
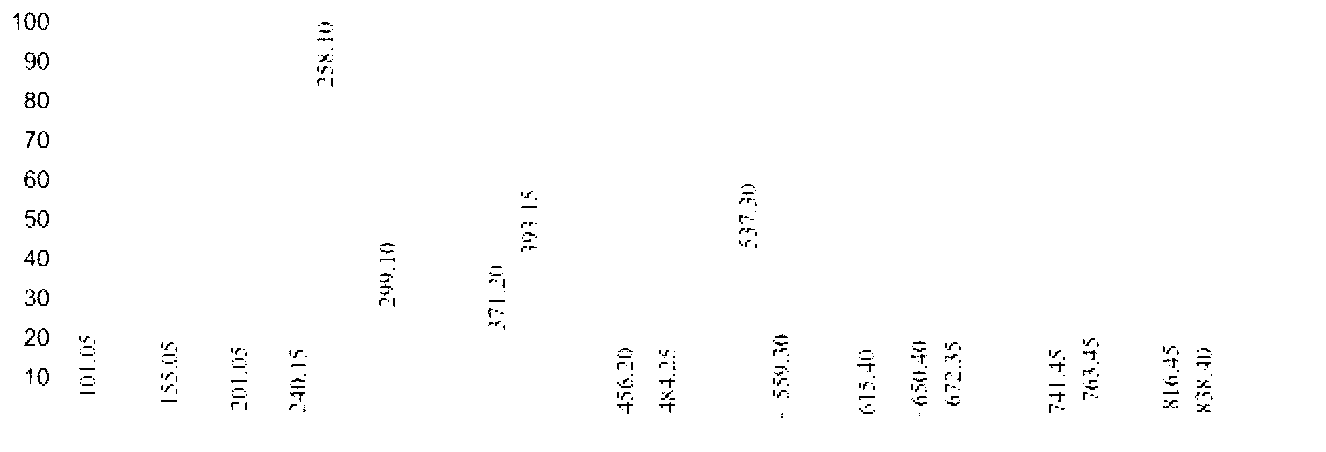
[0047] 17.26 parts of n-decanoic acid in Example 1 were changed to 14.42 parts of n-octanoic acid, and other conditions were unchanged, the purity of the 6-n-octylamidohexyl hydroxamic acid obtained was 91.4%, and the yield was 93.2%. Product Mr: 272.21, detected by mass spectrometry MS: 258.10 [M-15] (see figure 2 ), is the peak of methyl removal. NMR analysis and infrared spectrum analysis spectra are shown in Image 6 with Figure 9 , see Table 1 and Table 2 for the data.
Embodiment 3
[0048] Embodiment 3: Preparation of 6-isooctylamidohexyl hydroxamic acid
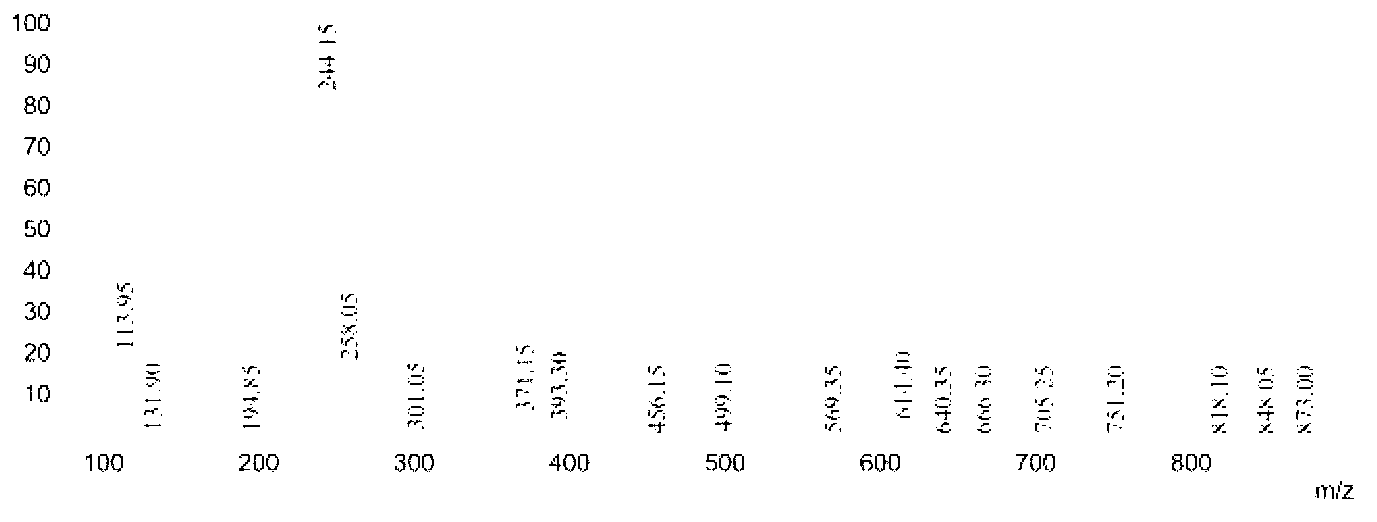
[0049] 17.26 parts of n-decanoic acid in Example 1 were changed to 14.42 parts of isooctanoic acid, and other conditions were unchanged, the purity of the obtained 6-isooctylamidohexyl hydroxamic acid was 89.4%, and the yield was 91.2%. Product Mr: 272.21, detected by mass spectrometry MS: 244.15 [M-29] (see image 3 ), is the peak of dropping the branched ethyl group; 258.05[M-15] is the peak of dropping the methyl group. NMR analysis spectrum see Figure 7 , see Table 2 for the data.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com