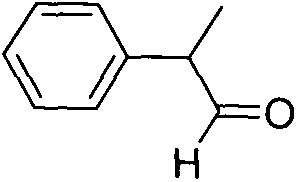
Preparation method of hydratropic aldehyde
A technology of Solanum aldehyde and Solanum aldehyde sulfonate, which is applied in the preparation of heterocyclic compounds, the separation/purification of carbonyl compounds, organic chemistry, etc., can solve the problems of difficult availability of raw materials and restrictions on industrial use, and achieve easy industrialization , low cost, and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
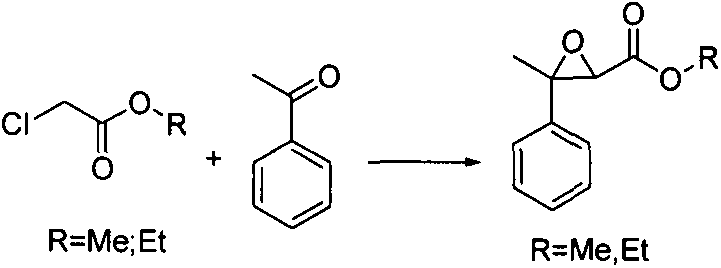
[0031] Weigh 5000g of 20% sodium methoxide into a 10L reaction flask, control the temperature down to 20°C, add 1200g of acetophenone into a 10L reaction flask, slowly add 1.5Kg of methyl chloroacetate dropwise, after 1h of dropwise addition, stir for 2h, The results showed that the reaction was basically complete. Ethanol is removed, and rectification under reduced pressure is obtained to obtain 3-methyl-3-phenylglycidic acid methyl ester with a content ≥ 98%, and the yield is 70-80%.
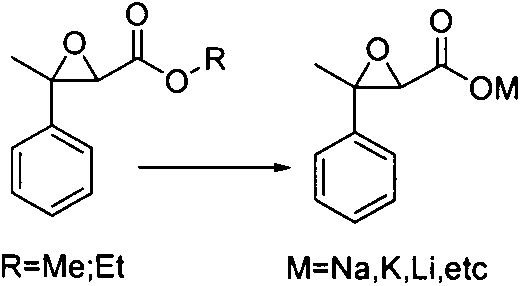
[0032] In a three-necked flask equipped with a thermometer, stirring, and 10L, add 1kg of the above-mentioned 3-methyl-3-phenyl glycidic acid methyl ester, 1L of 20% aqueous sodium hydroxide solution, stir the reaction, and follow the reaction by chromatography. Until the glyceride is completely converted, the reaction time is about 4 hours and the 3-methyl-3-phenylglycidyl sodium salt is obtained by filtration with a yield of 90-100%.
[0033] Add 1000 g of the above-prepared 3-methyl-3-phen...
Embodiment 2
[0037] Weigh 3000g of 20% sodium ethoxide into a 10L reaction flask, control the temperature down to 10°C, add 1500g of acetophenone into a 10L reaction flask, slowly add 1.5Kg of ethyl chloroacetate dropwise, after 1h of dropwise addition, stir for 2h, Sent to GC for detection, the results showed that the reaction was basically complete. Ethanol is removed, and 3-methyl-3-phenyl glycidic acid ethyl ester with a content ≥ 98% is obtained by rectification under reduced pressure, and the yield is 70-80%.
[0038] In a three-necked flask equipped with a thermometer, stirring, and 10L, add 1kg of the above-mentioned 3-methyl-3-phenyl glycidic acid ethyl ester, 1L of 20% aqueous sodium hydroxide solution, stir the reaction, and follow the reaction by chromatography. Until the ethyl glycerate is completely converted, the reaction time is about 10 hours and the 3-methyl-3-phenylglycidyl sodium salt is obtained by filtration with a yield of 90-100%.
[0039] In a three-neck flask equip...
Embodiment 3
[0043] Weigh 3600g of 20% sodium isopropoxide into a 10L reaction flask, control the temperature down to 40°C, add 1200g of acetophenone into a 10L reaction flask, slowly add 2.4Kg of ethyl chloroacetate dropwise, after 1 hour of dropwise addition, stir 5h, sent for detection, the results showed that the reaction was basically complete. Remove ethanol, rectify under reduced pressure (1mmHg) to obtain 3-methyl-3-phenyl glycidic acid ethyl ester with a content ≥ 98%, and the yield is 70-80%.
[0044] In a three-necked flask equipped with a thermometer, stirring, and 10L, add 1kg of the above-mentioned 3-methyl-3-phenyl glycidic acid ethyl ester, 1L of 20% aqueous sodium hydroxide solution, stir the reaction, and follow the reaction by chromatography. Until the ethyl glycerate was completely converted, the reaction time was about 24 hours and the sodium salt of 3-methyl-3-phenylglycidyl was obtained by filtration with a yield of 90-100%.
[0045] Add 1 kg of the above-prepared 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com