Preparation method of 2-(1-adamantly) glyoxylic acid
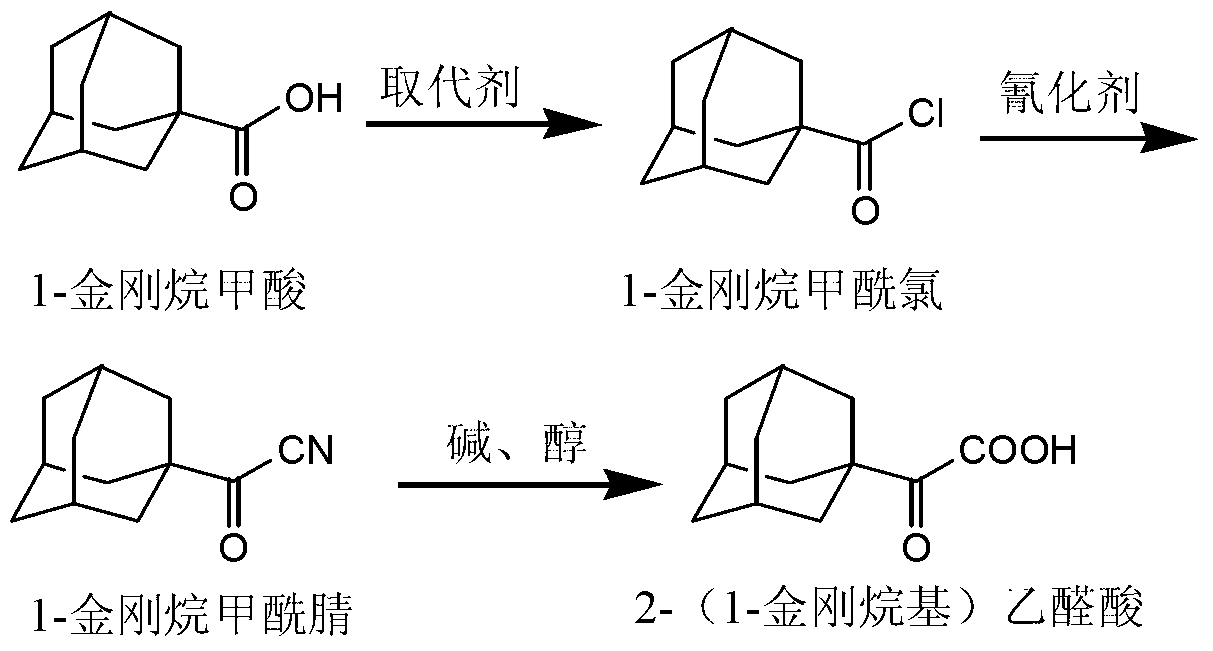
A technology of adamantyl and adamantane carboxylic acid is applied in the preparation of saxagliptin intermediates and the field of preparation of saxagliptin intermediates 2-(1-adamantyl)glyoxylic acid, which can solve the complicated synthesis steps , large product loss, low yield and other problems, to achieve the effect of simple post-processing operation, short reaction time, high yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
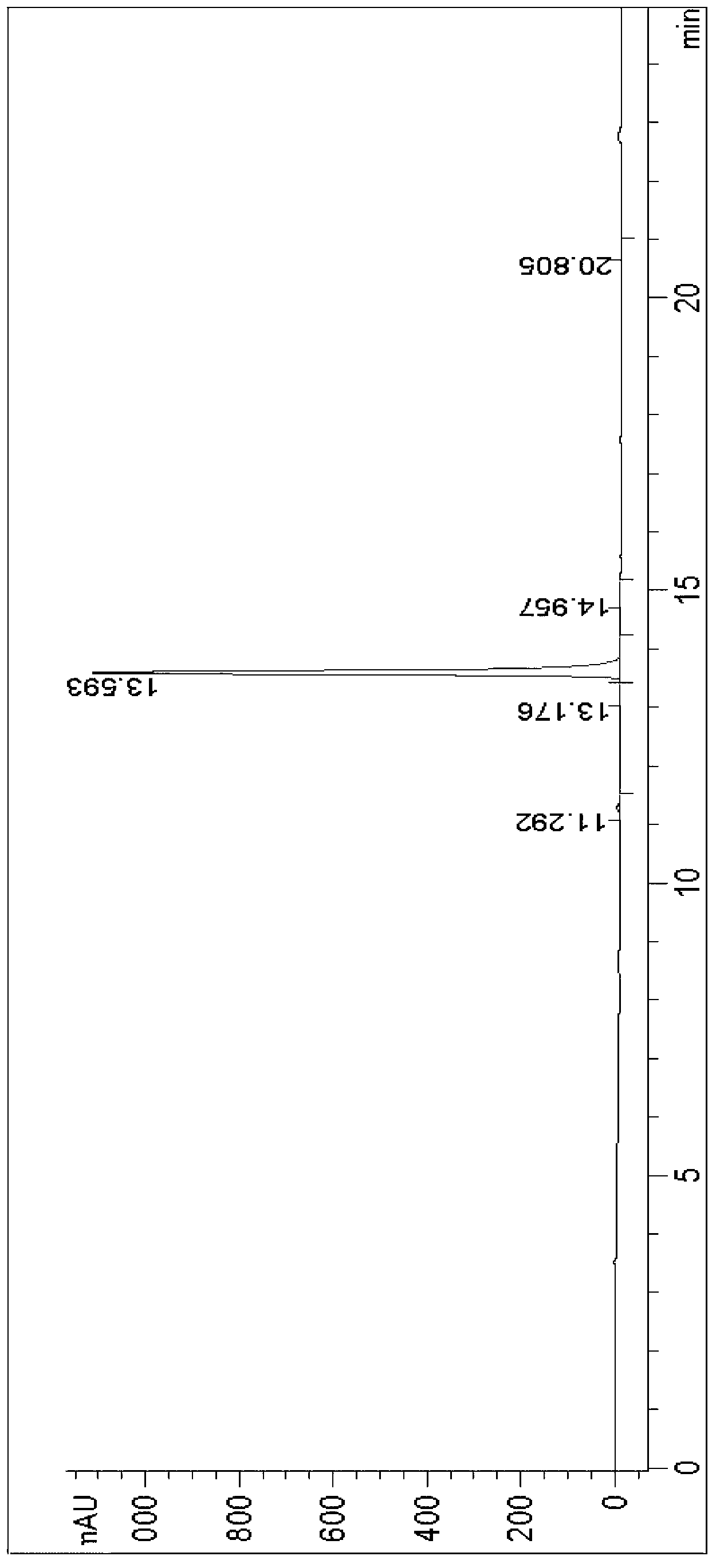
Image
Examples
Embodiment 1
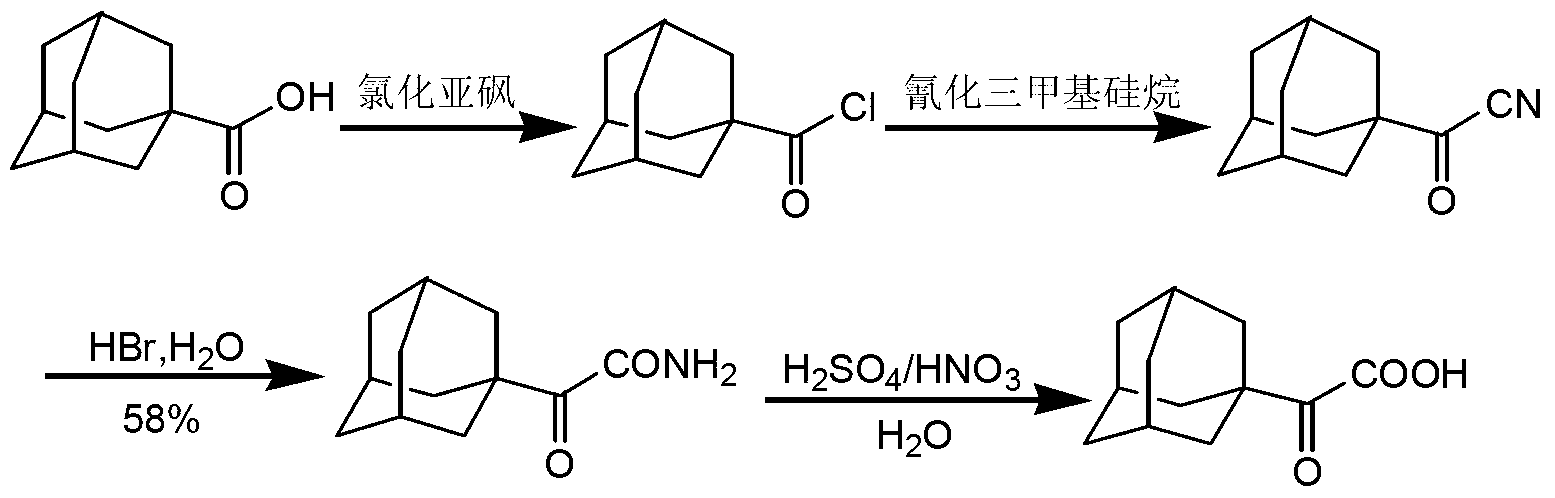
[0033] Substitution reaction: Add 50 g of 1-adamantanecarboxylic acid into a reaction flask and dissolve in 300 mL of dichloromethane, and stir until all 1-adamantanecarboxylic acid is dissolved to obtain a reaction solution. The reaction liquid was cooled to 0° C., and 41.3 g of sulfuryl chloride was added to the reaction liquid. After the addition, the reaction liquid was heated to 25° C. and stirred for 2 hours until the reaction was complete. The reaction solution after the complete reaction was directly concentrated to obtain 55.1 g of 1-adamantanecarbonyl chloride with a yield of 100%.
[0034] Cyanide reaction: Add 55.1g of 1-adamantanecarbonyl chloride prepared above into the reaction flask, dissolve in 100mL of methyl tert-butyl ether and 20mL of N,N-dimethylformamide, stir until 1-adamantanecarbonyl chloride All dissolved in the reaction solution. Add 14.9 g of sodium cyanide in batches to the reaction solution, and continue stirring for 5 hours after the addition i...
Embodiment 2
[0037] Substitution reaction: add 45.1g of 1-adamantanecarboxylic acid into the reaction flask and dissolve in 300mL of chloroform, and stir until the 1-adamantanecarboxylic acid is completely dissolved to obtain a reaction solution. The reaction liquid was cooled to -2°C, and 35.1 g of oxalyl chloride was added to the reaction liquid. After the addition, the reaction liquid was heated to 22°C and stirred for 1.5 hours until the reaction was complete. The reaction solution after the complete reaction was directly concentrated to obtain 49.5 g of 1-adamantanecarbonyl chloride, and the yield was 100%.
[0038] Cyanogenation reaction: Add 49.5g of 1-adamantanecarbonyl chloride prepared above into the reaction flask, dissolve in 100mL ether and 20mL N,N-dimethylacetamide, stir until 1-adamantanecarbonyl chloride is completely dissolved to obtain a reaction solution . Add 17.6 g of potassium cyanide in batches to the reaction solution, and continue stirring for 5 hours after the a...
Embodiment 3
[0041] Substitution reaction: add 63.1g of 1-adamantanecarboxylic acid into the reaction flask and dissolve in 350mL of 1,2-dichloroethane, stir until all of the 1-adamantanecarboxylic acid is dissolved to obtain a reaction solution. The reaction solution was cooled to 0° C., and 63.2 g of phosphorus oxychloride was added to the reaction solution. After the addition, the reaction solution was heated to 20° C. and stirred for 2.5 hours until the reaction was complete. The reaction solution after the completion of the reaction was directly concentrated to obtain 69.5 g of 1-adamantanecarbonyl chloride, with a yield of 100%.
[0042] Cyanide reaction: Add 69.5g of 1-adamantanecarbonyl chloride prepared above into the reaction flask, dissolve in 120mL of isopropyl ether and 30mL of N,N-dimethylformamide, and stir until 1-adamantanecarbonyl chloride is completely dissolved to obtain The reaction solution. Add 68.9 g of cuprous cyanide in batches to the reaction solution, and conti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com