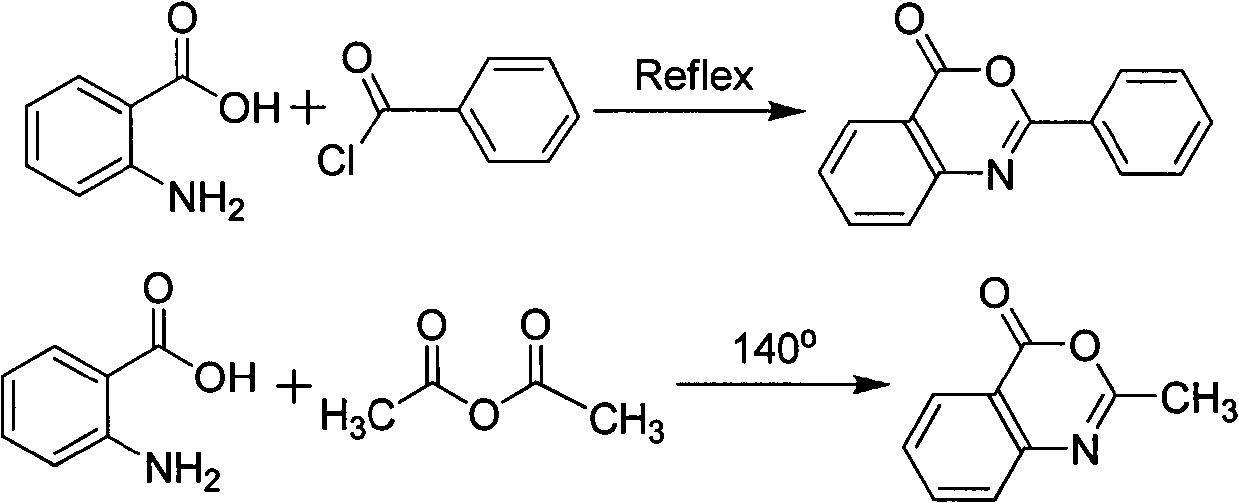
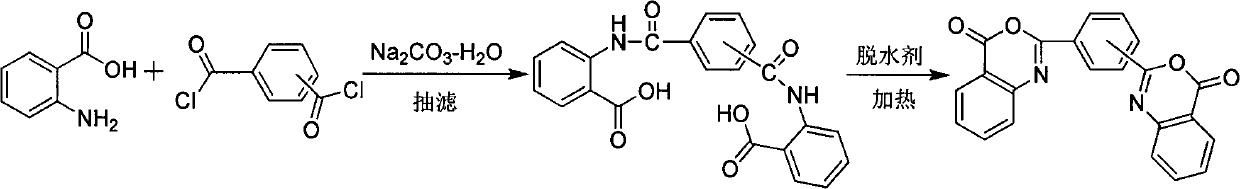
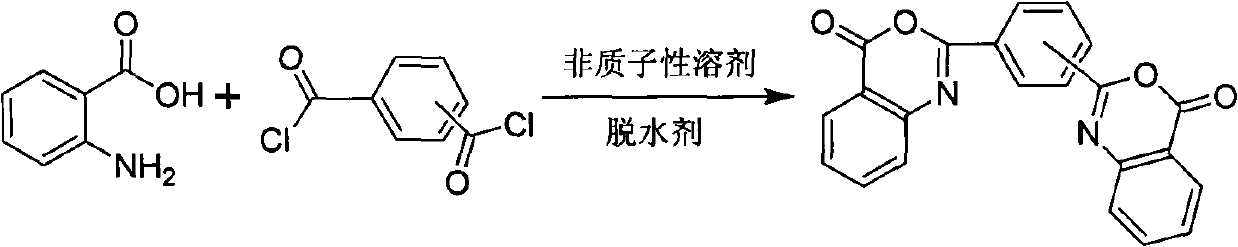
Novel synthetic method of bis-benzoxazine ketone ultraviolet absorbent
A technology of ultraviolet absorber and benzoxazine, which is applied in the synthesis field of bisbenzoxazinone ultraviolet absorbers, can solve the problems of high cost, complicated operation, low reaction yield and the like, and achieves low cost and simple equipment. , the effect of simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Dissolve 1.37g of anthranilic acid in 20mL of acetone, and drop 20mL of acetone solution containing 1.3g of terephthalic anhydride into the acetone solution of anthranilic acid under stirring within 1 hour, and stir at room temperature for three hours , add 2mL of concentrated sulfuric acid, reflux under stirring for 7 hours, cool down, filter after crystallization, collect light yellow crystals, wash once with 10mL of acetone, it is the ortho-dibenzoxazinone ultraviolet absorber, and the yield is 86%.
Embodiment 2
[0021] Dissolve 1.37g of anthranilic acid in 20mL of pyridine, 1.02g of isophthaloyl chloride in 20mL of pyridine, and drop the pyridine solution of isophthaloyl chloride into the pyridine solution of anthranilic acid within 1 hour under stirring , stirred at room temperature for one hour, then added benzene to reflux to separate water, stopped heating until no water was produced, cooled to obtain a white yellowish solid, filtered, washed once with acetone, and it was meta-bisbenzoxazinone UV absorber, with a yield of 48 %.
Embodiment 3
[0023] Dissolve 1.37g of anthranilic acid in 20mL of pyridine, 1.02g of isophthaloyl chloride in 20mL of pyridine, and drop the pyridine solution of isophthaloyl chloride into the pyridine solution of anthranilic acid within 1 hour under stirring After stirring at room temperature for one hour, 10 g of calcium oxide was added to reflux, cooled after six hours to obtain a light yellow solid, filtered, washed once with acetone, washed with water, and dried to obtain meta-bisbenzoxazinone ultraviolet absorber with a yield of 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com