Sarafloxacin hydrochloride sustain-released injection and preparation method thereof
A technology for salafloxacin hydrochloride and injection, which can be applied in pharmaceutical formulations, emulsion delivery, medical preparations containing active ingredients, etc., can solve problems such as limitations, reduce labor intensity, reduce stress response, and improve clinical treatment effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
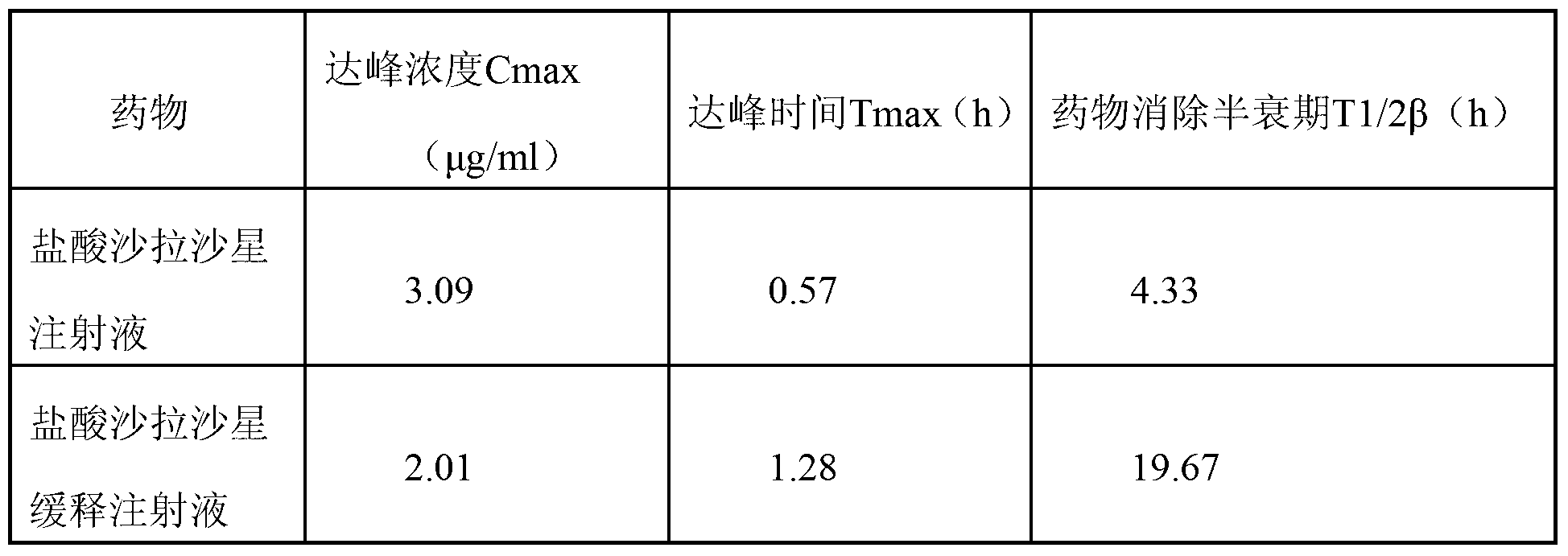
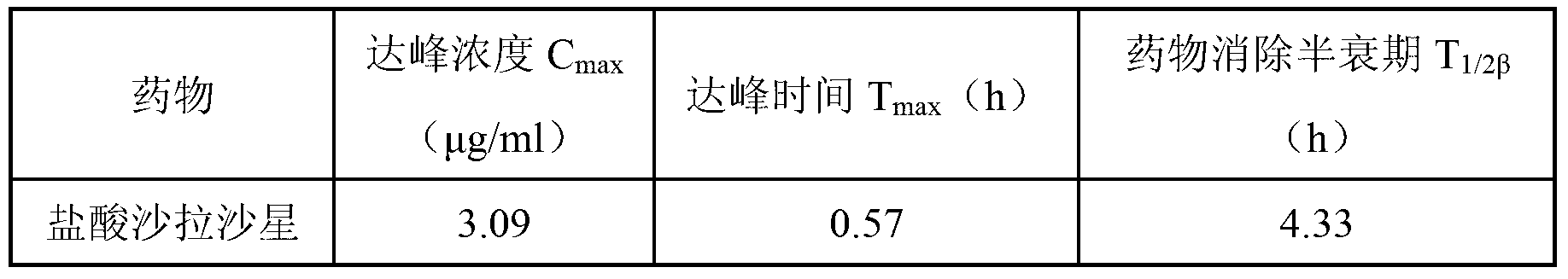
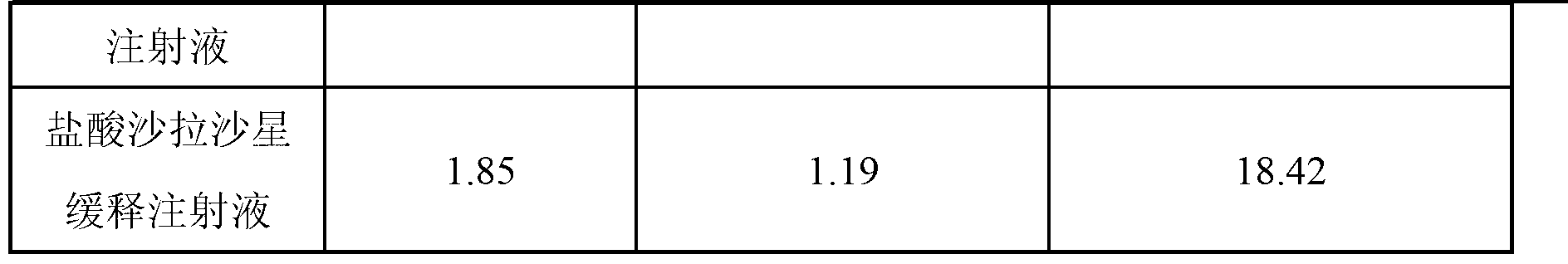
[0034] The formulation of sarafloxacin hydrochloride sustained-release injection is shown in Table 1.
[0035] The formula of table 1 sarafloxacin hydrochloride sustained-release injection
[0036] raw material name
Formula ratio
Sarafloxacin hydrochloride raw material (g)
5.0
Soybean oil for injection (g)
20.0
EDTA-2Na(g)
0.02
Glycerin (g)
4
Soy Lecithin (g)
3.50
Tween 80 (g)
3.0
VE (g)
0.15
Water for injection (g)
64.33
[0037] Preparation method of sarafloxacin hydrochloride sustained-release injection:
[0038] (1) Micronize sarafloxacin hydrochloride and pass through a 500-mesh sieve;
[0039] (2) Weigh sarafloxacin perhydrochloride raw material drug, VE, soybean oil for injection and soybean lecithin according to the prescription quantity, place it on a constant temperature magnetic stirrer and heat it to 70°C, and mix and stir evenly at a stirring speed of 900r...
Embodiment 2
[0053] The formula of sarafloxacin hydrochloride sustained-release injection is shown in Table 3.
[0054] The formula of table 3 sarafloxacin hydrochloride sustained-release injection
[0055] raw material name
Prescription ratio
Sarafloxacin hydrochloride raw material (g)
2.5
Soybean oil for injection (g)
15.0
EDTA-2Na(g)
0.03
Tween 80 (g)
2.5
CMC-Na(g)
0.2
VE (g)
0.02
Water for injection (g)
79.75
[0056] Preparation method of sarafloxacin hydrochloride sustained-release injection:
[0057] (1) Micronize sarafloxacin hydrochloride and pass through a 600-mesh sieve;
[0058] (2) Weigh sarafloxacin hydrochloride raw material drug, VE and soybean oil for injection according to the prescription quantity, place on a constant temperature magnetic stirrer and heat to 80°C, and mix and stir evenly at a stirring speed of 800r / min to obtain a mixture A .
[0059](3) Weigh CMC-Na acco...
Embodiment 3
[0078] The formula of sarafloxacin hydrochloride sustained-release injection is shown in Table 6.
[0079] Table 6 Sarafloxacin Hydrochloride Sustained Release Injection Formula
[0080] raw material name
Prescription ratio
Sarafloxacin hydrochloride raw material (g)
5.0
Corn oil for injection (g)
28
EDTA-2Na(g)
0.04
Soy Lecithin (g)
5.0
CMC-Na(g)
0.2
VE (g)
0.1
Water for injection (g)
61.66
[0081] Preparation method of sarafloxacin hydrochloride sustained-release injection:
[0082] (1) Micronize sarafloxacin hydrochloride and pass through a 400-mesh sieve;
[0083] (2) Weigh sarafloxacin hydrochloride raw material drug, corn oil for injection, soybean lecithin and VE according to the prescription quantity, heat it on a constant temperature magnetic stirrer to 60°C, and mix and stir evenly at a stirring speed of 1200r / min to obtain Mixture A;
[0084] (3) Weigh CMC-Na accord...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com