Sarafloxacin hydrochloride clathrate and preparation method thereof
A technology of sarafloxacin hydrochloride and inclusion compound, which is applied in the field of medicine, can solve the problems of no sarafloxacin hydrochloride inclusion compound, etc., and achieve the effect of easy operation and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 6.4g of sarafloxacin hydrochloride, dissolve it in 100ml of 0.02M sodium hydroxide solution, ultrasonicate for 15min in a 60°C water bath at 40KHz, and weigh CH 3 -Add 20.0g of β-CD into sarafloxacin hydrochloride solution, heat and stir at 35°C for 3 hours, add 6.4g of polyvinylpyrrolidone into CH 3 - in β-CD sarafloxacin hydrochloride solution, grind mechanically for 30min, filter with suction, place in a freeze dryer at -30°C, and freeze-dry for 24h to obtain sarafloxacin hydrochloride CH 3 -β-CD clathrate.
[0030] The solubility of sarafloxacin hydrochloride is 0.074mg / mL, and the sarafloxacin hydrochloride CH prepared in Example 1 3 The solubility of -β-CD inclusion complex was 1.71mg / mL, the solubility increased by 23.1 times, the drug loading was 18.1%, and the inclusion rate was 85.9%.
Embodiment 2
[0032] Weigh 5.1g of sarafloxacin hydrochloride, dissolve it in 100ml of 0.03M sodium hydroxide solution, and ultrasonicate for 30min in a 50°C water bath at 38Hz; add 20.0g of HP-β-CD into the sarafloxacin hydrochloride solution, heat Stir, the temperature is 35°C, the time is 2 hours, add polyethylene glycol 6000 25.5g into the HP-β-CD sarafloxacin hydrochloride solution, mechanically grind for 20min, filter with suction, put it in a freeze dryer at -35°C, freeze After drying for 20 hours, the inclusion complex of sarafloxacin hydrochloride HP-β-CD is obtained.
[0033] The solubility of sarafloxacin hydrochloride is 0.074mg / mL, the solubility of the sarafloxacin hydrochloride HP-β-CD inclusion compound prepared in Example 2 is 1.79mg / mL, the solubility is increased by 24.2 times, and the drug loading is 9.7% , The inclusion rate is 92.1%.
Embodiment 3
[0035] Weigh 5.0g of sarafloxacin hydrochloride, dissolve it in 100ml of 0.04M sodium hydroxide solution, and ultrasonicate for 25min in a 42KHz water bath at 40°C; add 10.0g of HP-β-CD into the sarafloxacin hydrochloride solution, heat Stir, the temperature is 30°C, the time is 3 hours, add 15g of hydroxypropylmethylcellulose into the HP-β-CD sarafloxacin hydrochloride solution, mechanically grind for 20min, filter with suction, put in a freeze dryer at -40°C, Freeze-dry for 16 hours to obtain sarafloxacin hydrochloride HP-β-CD inclusion compound.
[0036] The solubility of sarafloxacin hydrochloride is 0.074mg / mL, the solubility of the sarafloxacin hydrochloride HP-β-CD inclusion complex prepared in Example 3 is 1.63mg / mL, the solubility has increased by 22.0 times, and the drug loading is 15.3% , The inclusion rate is 88.6%.
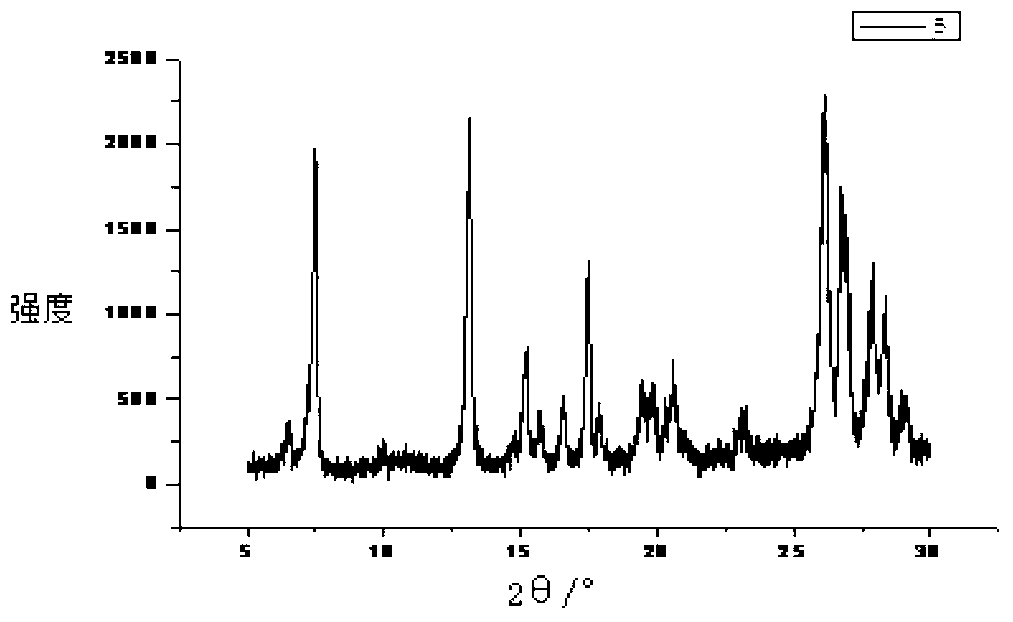
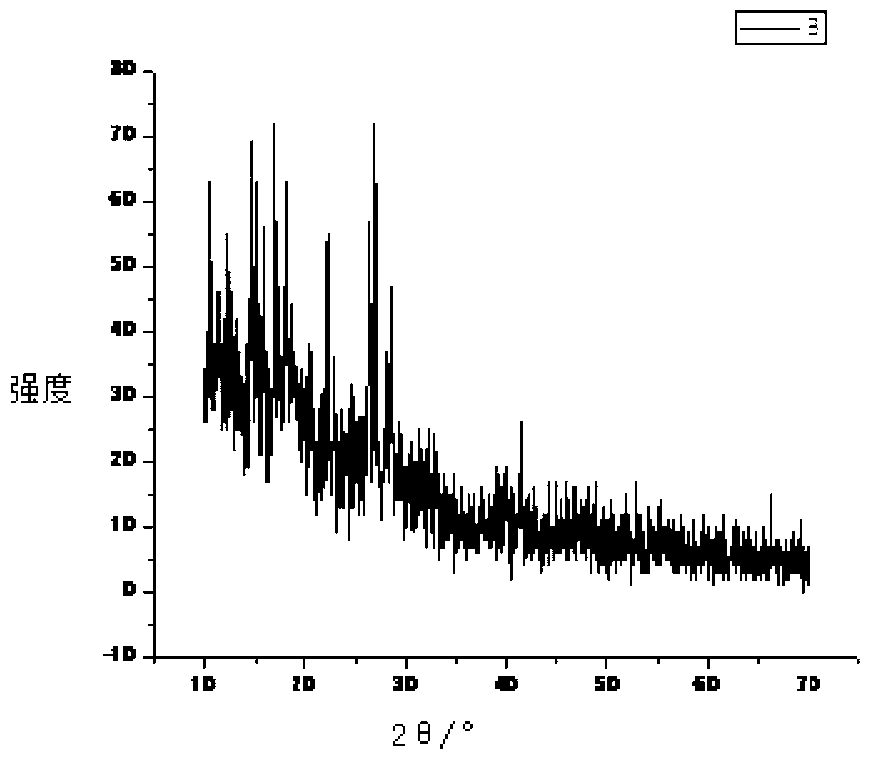
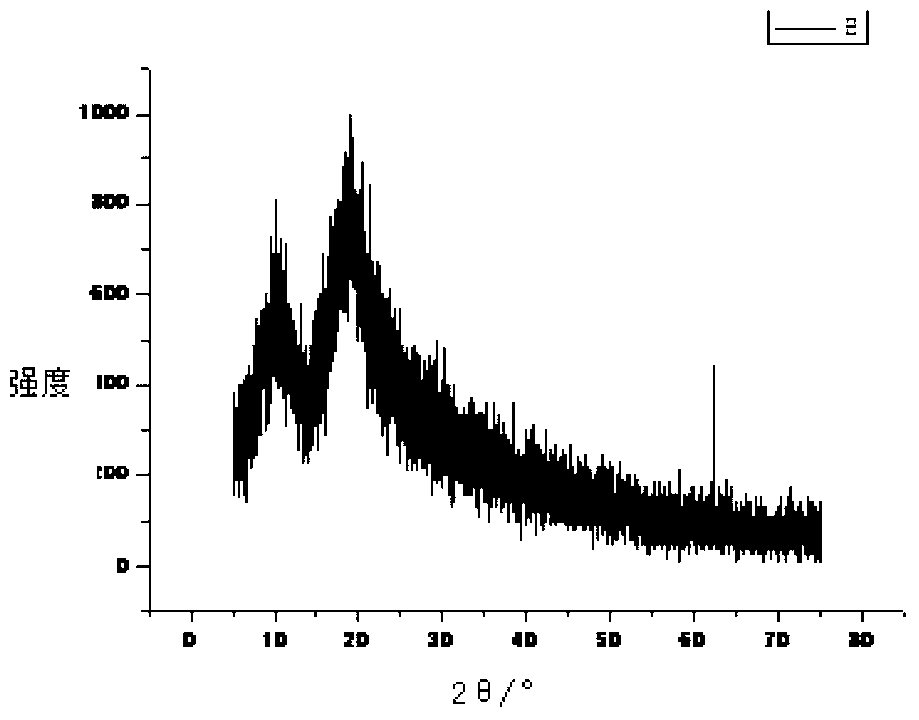
[0037] figure 1 The X-ray diffraction pattern of sarafloxacin hydrochloride shows that sarafloxacin hydrochloride has multiple specific crystal dif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com