A kind of synthetic method of pterostilbene sodium succinate
A technology of sodium stilbene succinate and a synthesis method, applied in the field of chemical medicine preparation, can solve the problems of unsuitability for large-scale industrial production, long operation time, complicated process and the like, and achieves the advantages of not becoming sticky, easy to operate, and good in reaction yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
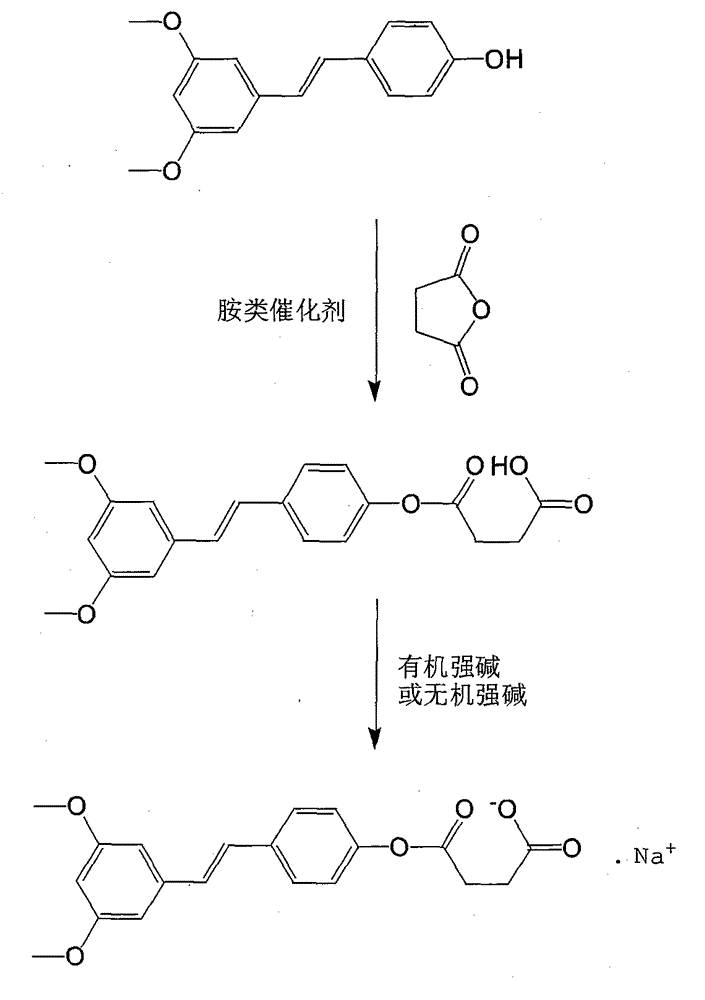
[0022] (1) Esterification reaction: In a 250ml dry three-necked flask, continuously feed N 2 . in N 2 Under protection, 20 g (0.078 mol) of pterostilbene, 10 g (0.1 mol) of succinic anhydride, and 150 ml of tetrahydrofuran were put into the reaction flask. Stir at room temperature until fully mixed and uniform, lower the temperature to 5°C, slowly add 14.4g (0.078mol) of tri-n-butylamine dropwise, after the drop is complete, stir the reaction at a temperature of 5-10°C, and confirm the end point of the reaction by tracking with TLC. After the reaction is completed, control the temperature at 45°C, concentrate under reduced pressure until no solvent is released, add 200ml of dichloromethane and 200ml of 0.1% hydrochloric acid solution for extraction, collect the organic phase, dry it with anhydrous sodium sulfate, concentrate under reduced pressure, and add a small amount of acetone By crystallization, 24.5 g of crude pterostilbene succinate half ester was obtained, with a yi...
Embodiment 2
[0025] (1) Esterification reaction: In a 250ml dry three-necked flask, continuously feed N 2 . in N 2 Under protection, 20 g (0.078 mol) of pterostilbene, 10 g (0.1 mol) of succinic anhydride, and 150 ml of 1,4-dioxane were put into the reaction flask. Stir at room temperature until fully mixed and uniform, lower the temperature to 5°C, slowly add 16.1g (0.078mol) of 1,3-dicyclohexylcarbodiimide dropwise, after the drop is complete, stir the reaction at a temperature of 5-10°C, and the TLC point Plate tracking confirms reaction endpoints. After the reaction is completed, control the temperature at 45°C, concentrate under reduced pressure until no solvent is released, add 200ml of dichloromethane and 200ml of 0.1% hydrochloric acid solution for extraction, collect the organic phase, dry it with anhydrous sodium sulfate, concentrate under reduced pressure, and add a small amount of acetone By crystallization, 20.5 g of crude pterostilbene succinate half ester was obtained, wi...
Embodiment 3
[0028] (1) Esterification reaction: In a 250ml dry three-necked flask, continuously feed N 2 . in N 2 Under protection, 20 g (0.078 mol) of pterostilbene, 10 g (0.1 mol) of succinic anhydride, and 150 ml of tetrahydrofuran were put into the reaction flask. Stir at room temperature until fully mixed evenly, lower the temperature to 5°C, slowly add 9.5g (0.078mol) of 4-dimethylaminopyridine dropwise, after the drop is complete, stir the reaction at a temperature of 5-10°C, and confirm the end point of the reaction by spotting the TLC plate . After the reaction is completed, control the temperature at 45°C, concentrate under reduced pressure until no solvent is released, add 200ml of dichloromethane and 200ml of 0.1% hydrochloric acid solution for extraction, collect the organic phase, dry it with anhydrous sodium sulfate, concentrate under reduced pressure, and add a small amount of acetone By crystallization, 18.4 g of crude pterostilbene succinate half ester was obtained, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com