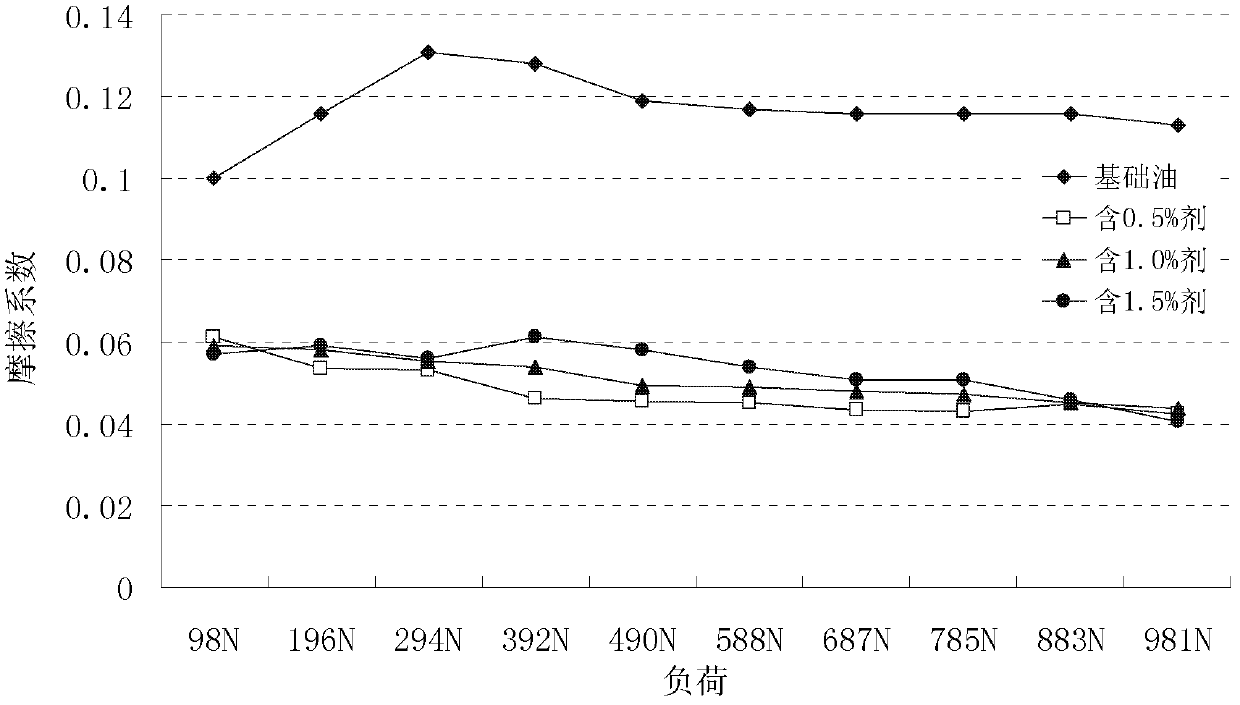
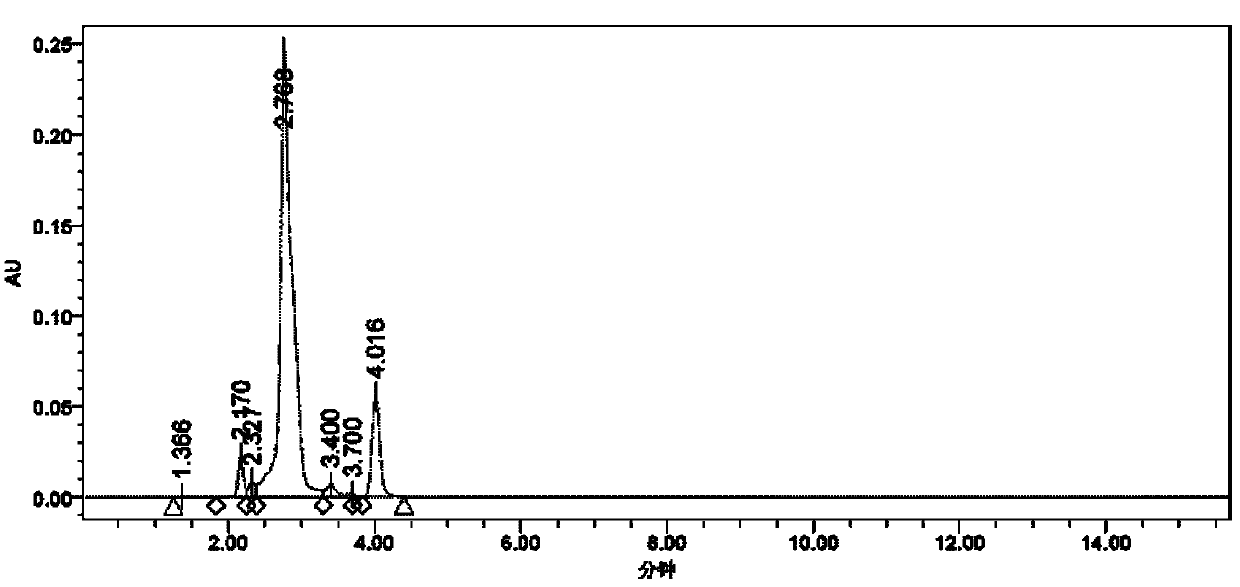
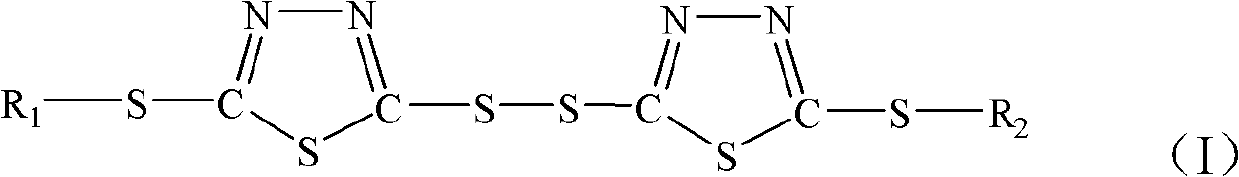
Double thiadiazole derivative and preparation method thereof
A technology of bisthiadiazole and derivatives, applied in lubricating compositions, petroleum industry, additives, etc., to achieve the effects of good solubility and excellent extreme pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Add 15.0g of 2,5-dimercapto-1,3,4-thiadiazole (0.1mol) and 80ml of ethyl acetate into a 500ml four-neck flask equipped with a reflux condenser and a thermometer, stir to dissolve, and add dropwise at 20°C 4.0g NaOH (0.1mol) aqueous solution 20mL, dropwise time is 10min, then add 0.3g tetrabutylammonium bromide (0.001mol) as catalyst, drop 13.7g (0.1mol) bromobutane at 20°C, The dropping time is 30min. After the dropping is completed, the temperature is gradually raised to 76°C for reflux reaction for 4h. After the reaction is completed, it is cooled to room temperature and filtered to obtain light yellow solid powder 2-thiobutyl-5-mercapto-1,3,4 - Thiadiazoles.
Embodiment 2
[0040] Add 15.0g of 2,5-dimercapto-1,3,4-thiadiazole (0.1mol) and 80ml of ethanol into a 500ml four-neck flask equipped with a reflux condenser and a thermometer, stir to dissolve, and add 4.4g of NaOH dropwise at 20°C (0.11mol) aqueous solution 21mL, dropwise time is 20min, then add 0.3g tetrabutylammonium bromide (0.001mol) as catalyst, drop 14.9g (0.1mol) chlorooctane at 20 ℃, dropwise time After the dropwise addition, gradually raise the temperature to 76°C for reflux reaction for 6 hours. After the reaction, cool to room temperature and filter to obtain light yellow solid powder 2-thiooctyl-5-mercapto-1,3,4-thiadiol azole.
Embodiment 3
[0042] Add 20.5g of 2-thiobutyl-5-mercapto-1,3,4-thiadiazole (0.1mol) synthesized in Example 1 in a 500ml four-necked flask equipped with a reflux condenser and a thermometer, and use 75ml of distilled water as The solvent was stirred and reacted, 13.6g of oxidant 0.12mol hydrogen peroxide (30%) was added dropwise, the dropping time was controlled at 10-15min, and then the temperature was raised to reflux for 6h. After the reaction, the product was extracted with petroleum ether, poured out and washed with water To neutrality, dry with anhydrous sodium sulfate and distill off the solvent to obtain a light yellow liquid product bis-5,5'-dithiobis-(1,3,4-thiadiazole-2-thiobutyl) additive. The S content of the product is 45.44%, and the theoretical S content is 46.82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com