Sulfhydrylated folic acid and preparation method thereof
A technology for sulfhydrylation and folic acid, which is applied in the field of sulfhydrylation folic acid and its preparation, and achieves the effects of convenient use, simple preparation process and broad application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of Disulfides by Oxidative Coupling of 3-Mercaptopropanol
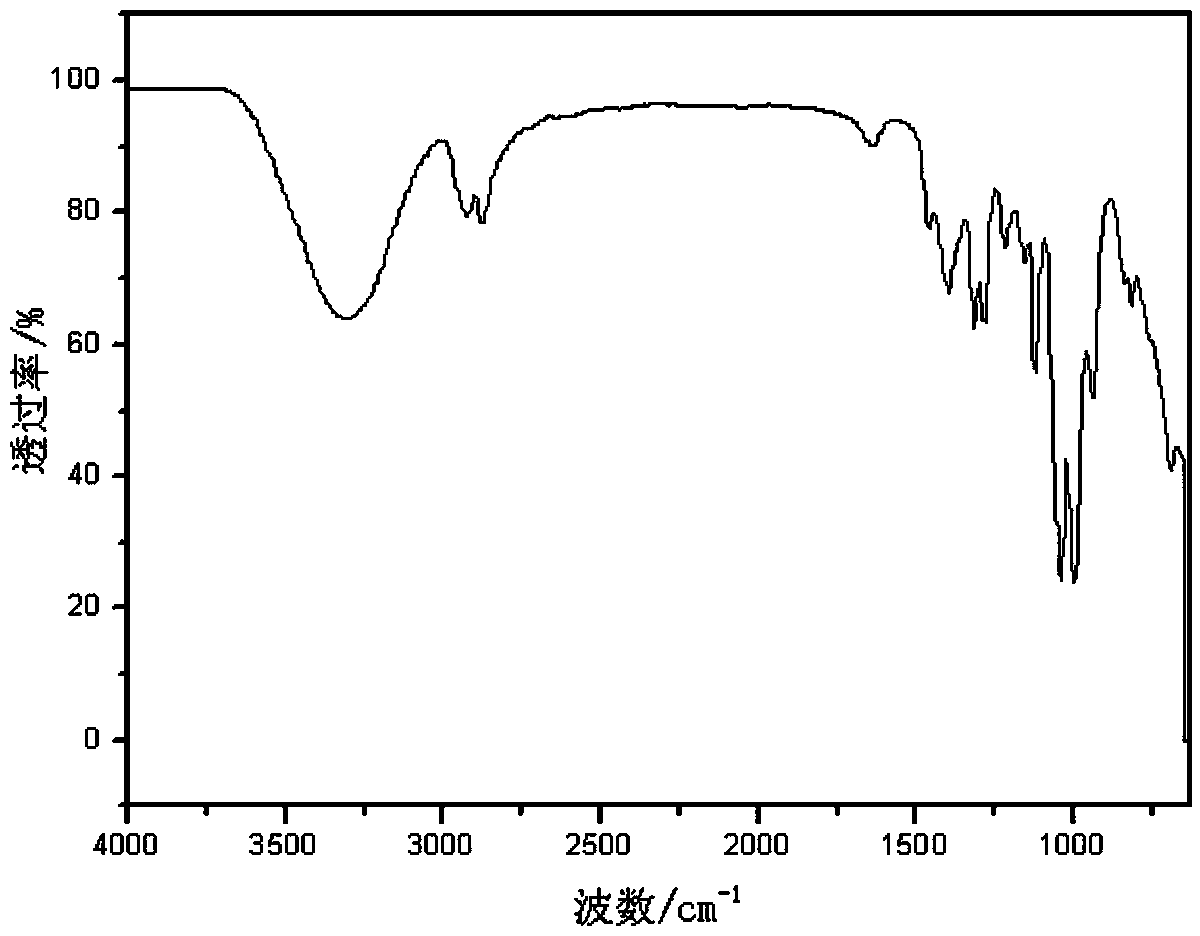
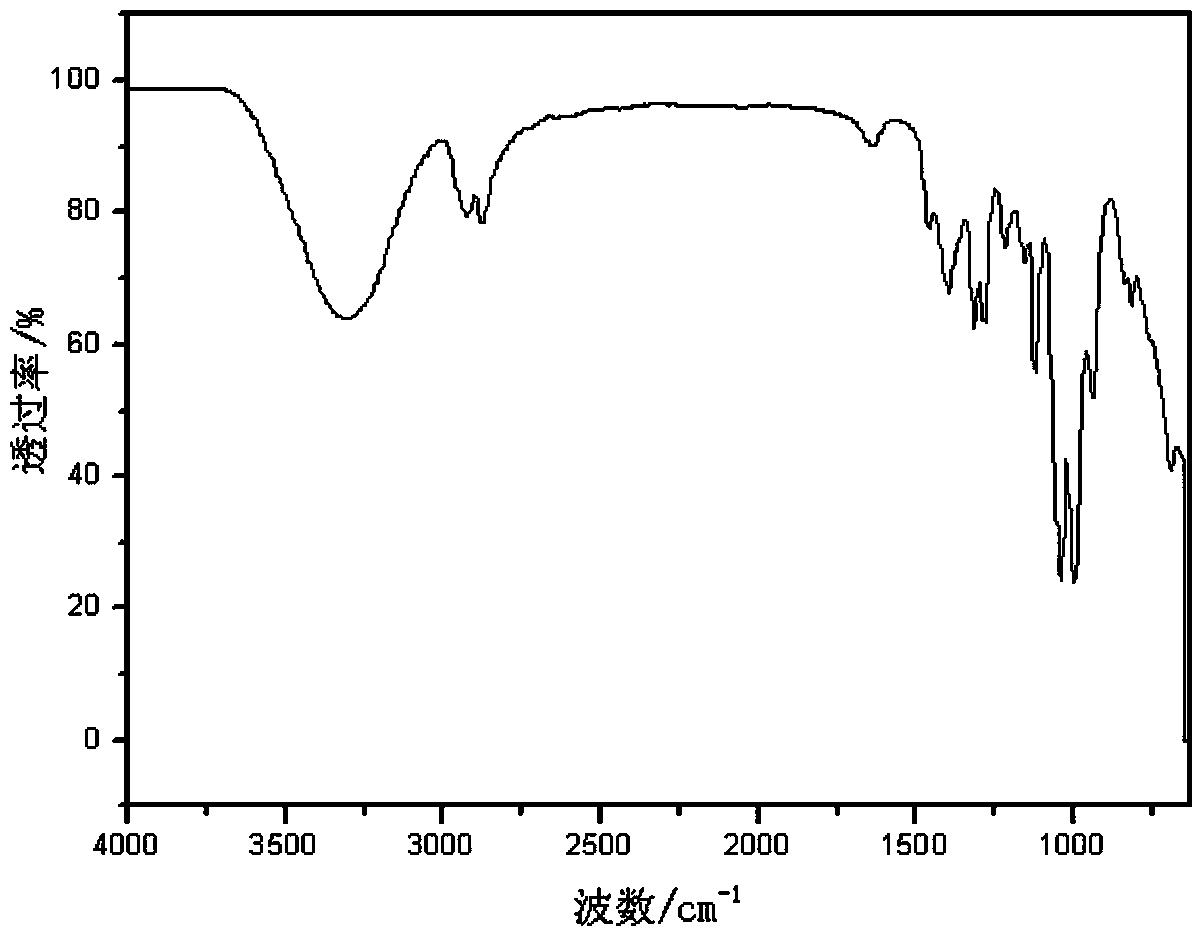
[0046] Add 9.216 g (0.100 mol, 2.50 mol / L) of 3-mercaptopropanol ethanol solution into a 100 mL Erlenmeyer flask equipped with a magnet and constant pressure funnel, and add 30% hydrogen peroxide 11.337 g (0.100 mol), put the Erlenmeyer flask into the ice-water mixture, add hydrogen peroxide dropwise to the solution in the Erlenmeyer flask, and stir for 2 hours. After the reaction, put the flask containing the mixed solution into the rotary evaporator, set the temperature at 80 °C, stop the rotary evaporation until the solution no longer produces bubbles, and the remaining liquid is the prepared disulfide 3,3'-dimercapto dipropanol. Use infrared spectroscopy to test and characterize 3-mercaptopropanol and product 3,3'-dimercaptodipropanol, see the attached figure 1 , figure 2 . It can be seen from the infrared spectrum of the product that the characteristic infrared absorption peak of the sulfh...
Embodiment 2
[0048] Preparation of Disulfides by Oxidative Coupling of 2-Mercapto-3-Butanol
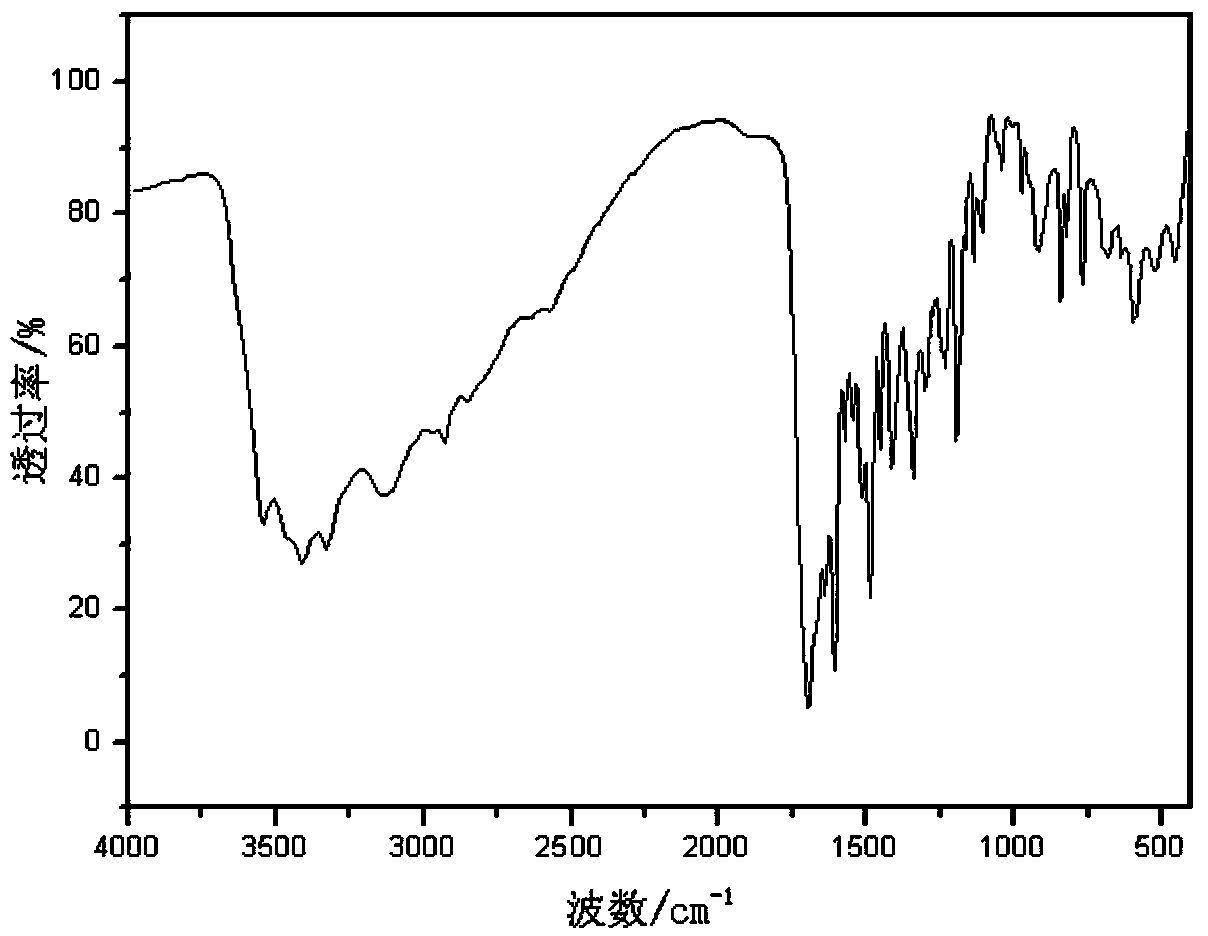
[0049] Add 7.433 g (0.070 mol, 2.30 mol / L) butanone solution of 2-mercapto-3-butanol into a 100 mL Erlenmeyer flask equipped with a magnet and a constant pressure funnel, and add a concentration of 30 % hydrogen peroxide 3.968 g (0.035 mol), put the Erlenmeyer flask into a low-temperature thermostat, control the reaction temperature at 10°C, add hydrogen peroxide dropwise to the solution in the Erlenmeyer flask, and stir for 4 hours. After the reaction was completed, 1.988 g (0.014 mol) of anhydrous sodium sulfate was added, the anhydrous sodium sulfate absorbed water and crystallized, and the filtrate was collected by filtration to obtain the disulfide 2,2’-dimercaptodi-3-butanol. Using infrared spectroscopy to test and characterize 2-mercapto-3-butanol and the product 2,2'-dimercaptodi-3-butanol, it can be seen from the infrared spectrum of the product that the characteristic infrared absorptio...
Embodiment 3
[0051] Preparation of Disulfides by Oxidative Coupling of 3-Mercaptohexanol
[0052] Add 6.712 g (0.050 mol, 2.20 mol / L) of 3-mercaptohexanol in acetone solution into a 100 mL Erlenmeyer flask equipped with a magnet and constant pressure funnel, and add 30% hydrogen peroxide 11.337 g (0.100 mol), the Erlenmeyer flask was placed in a low-temperature thermostat, and the reaction temperature was controlled at 10°C. Hydrogen peroxide was added dropwise to the solution in the Erlenmeyer flask, and the reaction was stirred for 6 hours. After the reaction was completed, the mixed solution was distilled under reduced pressure, and then rotary evaporated at a temperature of 80°C until the solution in the flask stopped bubbling to obtain the disulfide 3,3'-dimercaptodihexanol. Using infrared spectroscopy to test and characterize 3-mercaptohexanol and the product 3,3’-dimercaptodihexanol, it can be seen from the infrared spectrum of the product that the characteristic infrared absorption...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com