Antibacterial peptide, preparation method and applications thereof
An antibacterial peptide and amino acid technology, applied in the fields of biotechnology and genetic engineering, can solve problems such as unsatisfactory antibacterial activity, and achieve the effect of enhancing bactericidal activity in vitro and in vivo and easy to prepare
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
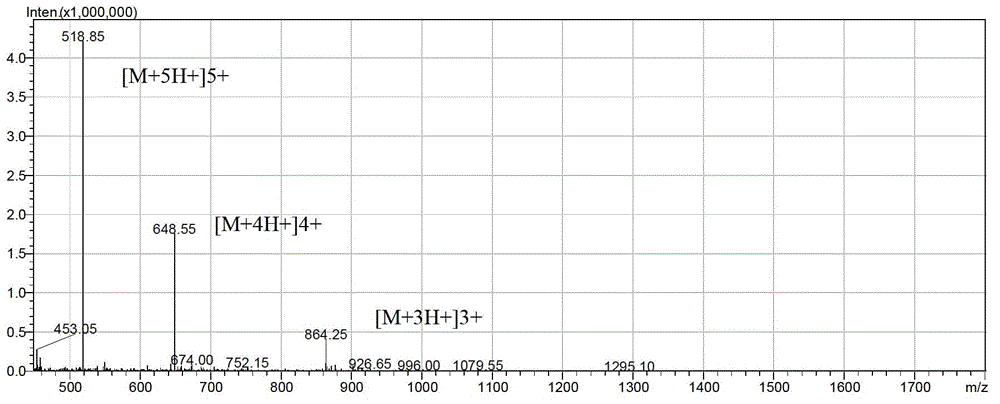
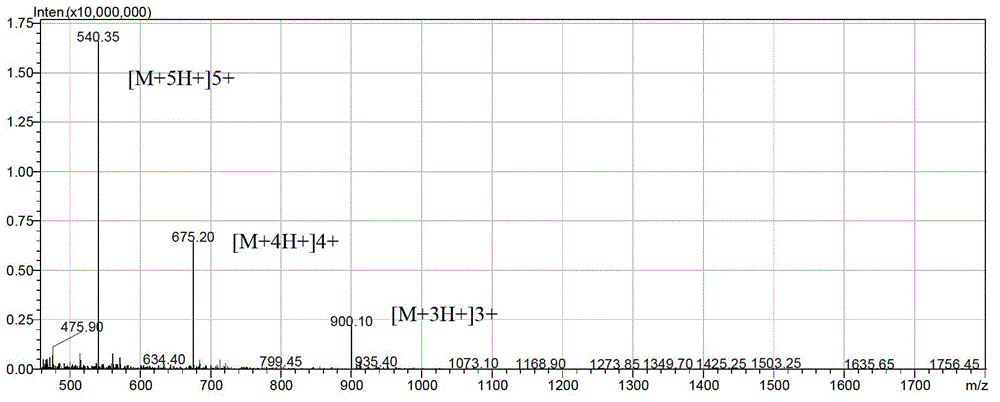
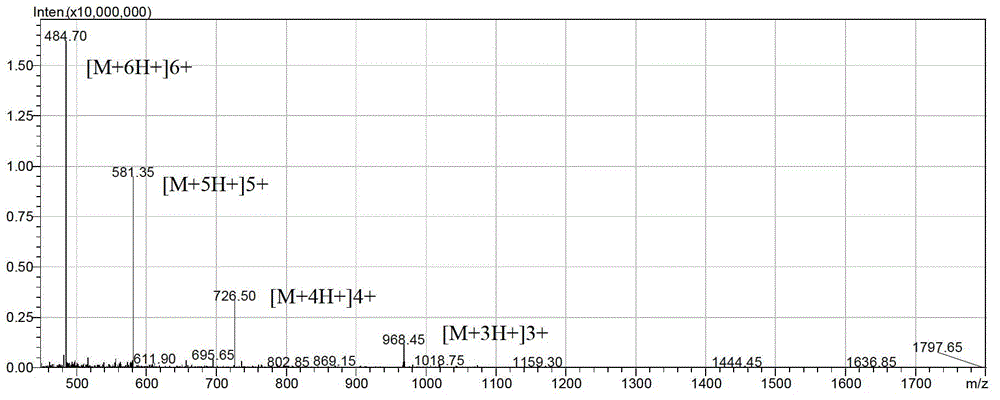
[0034] Example 1: Preparation and separation and purification of antimicrobial peptides
[0035] According to the above sequence, the antimicrobial peptide LFP-20 and the modified antimicrobial peptides LF-2 and LF-6 were synthesized by solid-phase chemical method using the Pioneer peptide synthesizer produced by Applied Biosystems. The solid-phase chemical method is automatically controlled by a peptide synthesizer, and the protected amino acids are coupled and deprotected one by one from the C-terminal to the N-terminal on the Fmoc-Arg (Pbf)-Wang Resin resin column in a pre-designed sequence. The synthesized peptide was cleaved by high-concentration TFA, then purified by reverse column, and the purified peptide was identified by mass spectrometry.
[0036] 1. Preparation of antimicrobial peptides (taking the preparation of 1mg LFP-20 as an example)
[0037]All the following reagents for the preparation of antimicrobial peptides were purchased from Applied Biosystems. The p...
Embodiment 2
[0050] Embodiment two: Bactericidal activity detection
[0051] Various bacterial strains used in the following examples were purchased from China Institute for the Control of Biological Products. The bactericidal activity of the antimicrobial peptides was detected by the improved micro broth dilution method to evaluate the bactericidal activities of the two modified antimicrobial peptides in the present invention.
[0052] Determination of antimicrobial peptide bactericidal activity was carried out according to the following steps: thaw the strain frozen at -80°C in ice, streak it on the MH agar plate with an inoculation loop, and culture it at 37°C to form a single colony; the single colony was inoculated on fresh MH In the broth medium, 37°C constant temperature shaking overnight culture activation; take the above overnight culture suspension and transfer it to fresh MH broth medium at a ratio of 1:100, and culture at 37°C 250rpm constant temperature shaking until OD 600nm...
Embodiment 3
[0055] Embodiment three: Sterilization speed detection
[0056] Set the concentration of the detected peptide according to the needs of the experiment, and generally use integer multiples of the MIC for detection. This experiment intends to measure the bactericidal curve of LFP-20 and its modified antimicrobial peptides against bacteria under the MIC concentration; the sampling time points are set at 0, 5, 10, 20, 30, 40, 50, 60, 90 , 120, 150, 180 and 240min. Add 1mL of the prepared bacterial suspension into the centrifuge tube, and at the same time, add the corresponding concentration of polypeptide to each tube, and set a blank control without polypeptide, and place it at 37°C for shaking culture; at the corresponding time point, take the bacterial suspension for appropriate dilution After overnight culture on MH agar plate medium, take 3 repeated total colony counts at a dilution concentration of 30-200 for colony counting, and calculate the bacterial concentration of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com