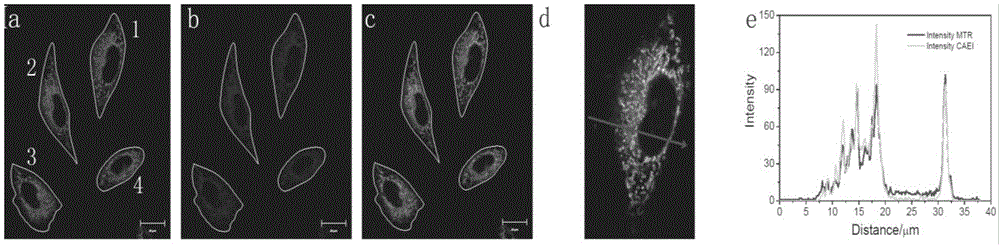
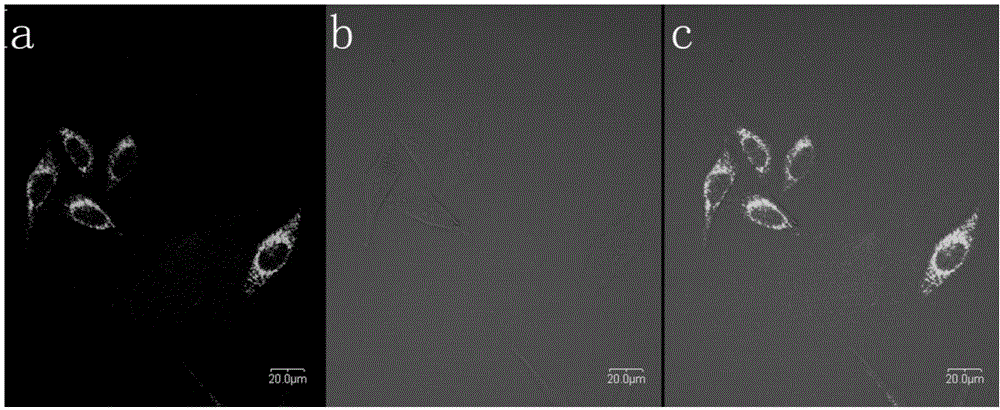
One and two-photon mitochondrion fluorescence probe and application thereof
A fluorescent probe and mitochondrial technology, applied in fluorescence/phosphorescence, luminescent materials, organic chemistry, etc., can solve the problems of lack of fluorescent probes and inapplicability of two-photon fluorescence microscopy, etc., and achieve good biocompatibility and two-photon performance Good, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of 9-ethylcarbazole (1a)
[0024] 28 g (500 mmol) of KOH was dissolved in 60 mL of acetone solution, and stirred at room temperature to obtain a yellow solution. Carbazole (13.2 g, 80 mmol) was added to the above solution, stirred at room temperature for 4 h to obtain a brown cloudy liquid. Acetone solution containing 1-bromoethane (9 mL, 120 mmol) was added gradually and reacted overnight. The reaction solution was poured into 1000 mL of water, a yellow precipitate was formed, and a yellow solid was obtained by filtration. The solid was recrystallized from ethanol to obtain a white solid, yield: 83%.
[0025] 1 H NMR (300MHz, CDCl 3 ),δ(ppm):8.10(d,J=7.8Hz,2H),7.39-7.49(m,4H),7.20-7.25(m,2H),4.36(q,J=7.2Hz,2H),1.43 (t, J=7.2Hz, 3H).
[0026] Synthesis of 9-Hydroxyethylcarbazole (1b)
[0027] The process is the same as above, and 9-hydroxyethyl carbazole can be synthesized with a yield of 81%. :
[0028] 1 H NMR (300MHz, CDCl 3 ),δ(ppm):8.79(d,J=7.8...
Embodiment 2
[0030] Synthesis of 3-acetyl-9-ethylcarbazole (2a)
[0031] 3.91g, 20mmol of compound 1a was dissolved in 75mL of anhydrous dichloromethane, and anhydrous AlCl3 (5.2g, 40mmol) was added under vigorous stirring to obtain a black precipitate. The mixture was cooled to 0°C under an ice bath, and acetic anhydride (2.04 g, 20 mmol) dissolved in 15 mL of anhydrous dichloromethane was added slowly. After the addition was complete, the ice bath was removed and stirred vigorously at room temperature overnight. The reaction solution was poured into a large amount of ice water, with NaHCO 3 Adjust the pH to 7-8. use CH 2 C1 2 Extraction, the organic layer was washed with water, anhydrous MgSO 4 Dry, filter and evaporate the solvent. The crude product was separated by column chromatography with petroleum ether / ethyl acetate (10:1) as the eluent to obtain a white solid with a yield of 87%.
[0032] 1 H NMR (400MHz, CDCl 3 ),δ(ppm):8.75(d,J=1.52Hz,1H),8.11-8.17(m,2H),7.50-7.54(m,1H...
Embodiment 3
[0037] Synthesis of 3-acetyl-6-bromo-9-ethylcarbazole (3a)
[0038] Under the protection of argon, compound 2 (2.2g, 9.85mmol) and NBS (1.9g, 10.67mmol) were added to 60mL of chloroform / glacial acetic acid mixed solution (1:1, v / v), and reacted at room temperature for 15h . After the reaction is complete, the reaction solution is poured into 500mL water, and the 2 Cl 2 extraction. The organic layer was washed with saturated NaCl solution, anhydrous MgSO 4Dry, filter and evaporate the solvent. The crude product was separated by column chromatography, using petroleum ether / ethyl acetate (10:1) as the eluent, to obtain a white flocculent solid, yield: 81%.
[0039] 1 H NMR (400MHz, CDCl 3 ),δ(ppm):8.71(d,J=1.52Hz,1H),8.30(d,J=1.88Hz,1H),8.18(dd,J 1 =8.70Hz,J 2 =1.70Hz,1H),7.62(dd,J 1 =8.64Hz,J 2 =1.92Hz,1H),7.44(d,J=8.68Hz,1H),7.35(d,J=8.64Hz,1H),4.4(q,J=7.25Hz,2H),2.74(s,3H), 1.47(t,J=7.26Hz,3H).
[0040] Synthesis of 6-bromo-9-ethylcarbazole (3b)
[0041] The proc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com