Compositions and methods for treatment of taupathy
一种蛋白病、治疗剂的技术,应用在用于治疗tau蛋白病的组合物和方法领域,能够解决癌细胞无特异性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0253] microbubble size
[0254] Experiments were carried out with gas-enriched fluids by using the diffuser of the present invention in order to determine the gas bubble size limit. Microbubble size limits were established by passing gas-enriched fluids through 0.22 and 0.1 micron filters. In performing these tests, a volume of fluid was passed through the diffuser of the present invention and a gas-enriched fluid was generated. Sixty milliliters of this fluid was drawn into a 60 ml syringe. The dissolved oxygen level of the fluid in the syringe was then measured by Winkler titration. The fluid in the syringe was injected through a 0.22 micron Millipore Millex GP50 filter into a 50 ml beaker. The dissolved oxygen rate of the material in the 50ml beaker was then measured. The experiment was carried out three times and the results shown in Table 4 below were obtained.
[0255] Table 4
[0256] Dissolved Oxygen in Syringe
Dissolved oxygen after 0.22 micron f...
Embodiment 2
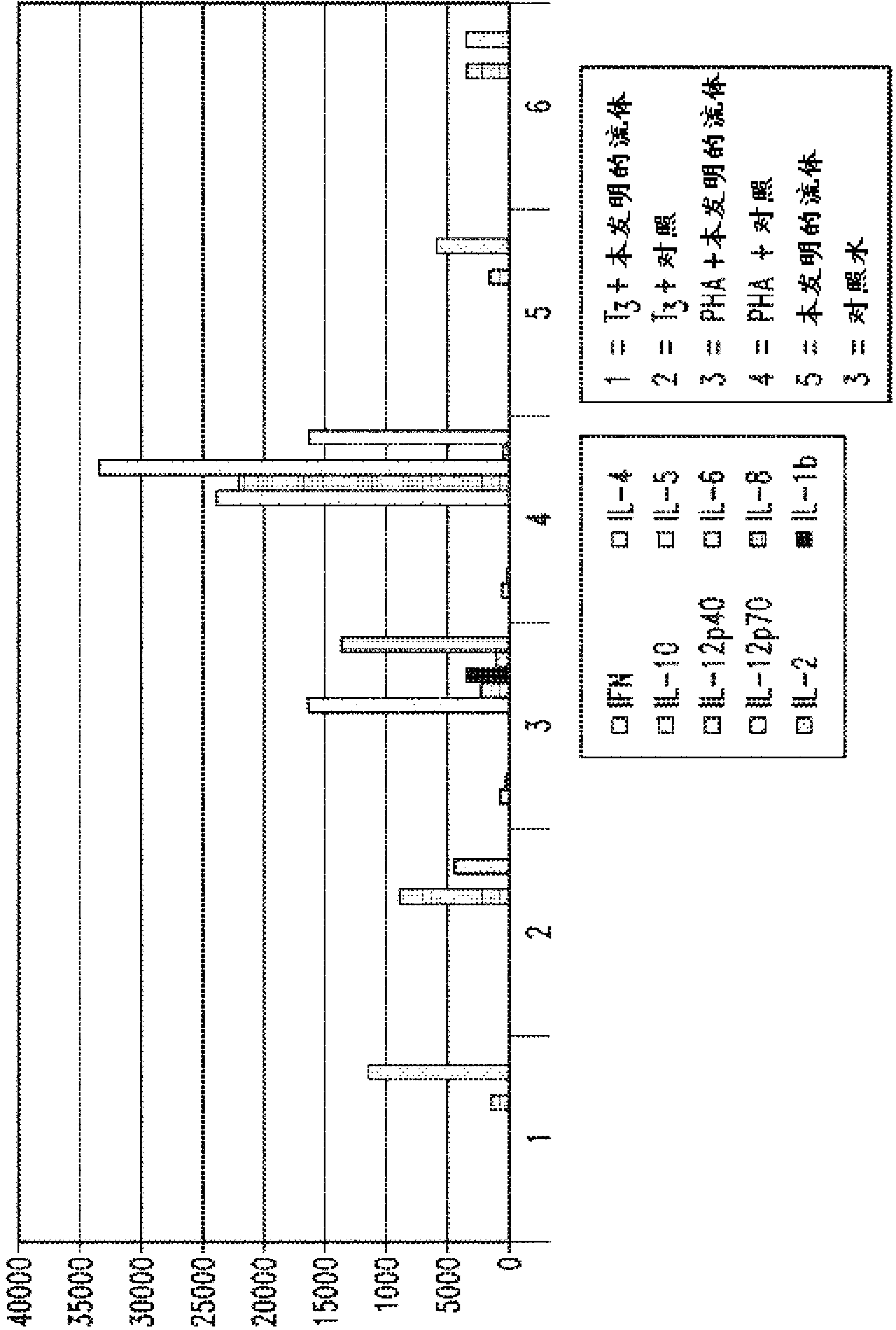
[0260] (cytokine profile determined)
[0261] Pooled lymphocytes were obtained from a healthy voluntary donor. The buffy coat samples were washed to remove platelets according to standard procedures. lymphocytes in 2x10 6 Concentrations per plate were inoculated into RPMI medium (+50 mm HEPES) diluted with gas-enriched fluid of the invention or distilled water (control). Cells were stimulated with 1 μg / ml T3 antigen or 1 μg / ml phytohemagglutinin (PHA) (pan T cell activator) or left unstimulated (recessive control). After 24 hours of incubation, cells were checked for viability and supernatants were extracted and frozen.
[0262] Thaw the supernatant, centrifuge, and use (Luminex) bead lite protocol and platform for testing cytokine expression.
[0263] Two million cells were seeded into 6 wells of a 24-well plate containing complete RPMI + 50 mm Hepes with oxygen-enriched fluid (water) of the invention (wells 1, 3 and 5) or distilled water ( Wells 2, 4 and 6) (dilute 10...
Embodiment 3
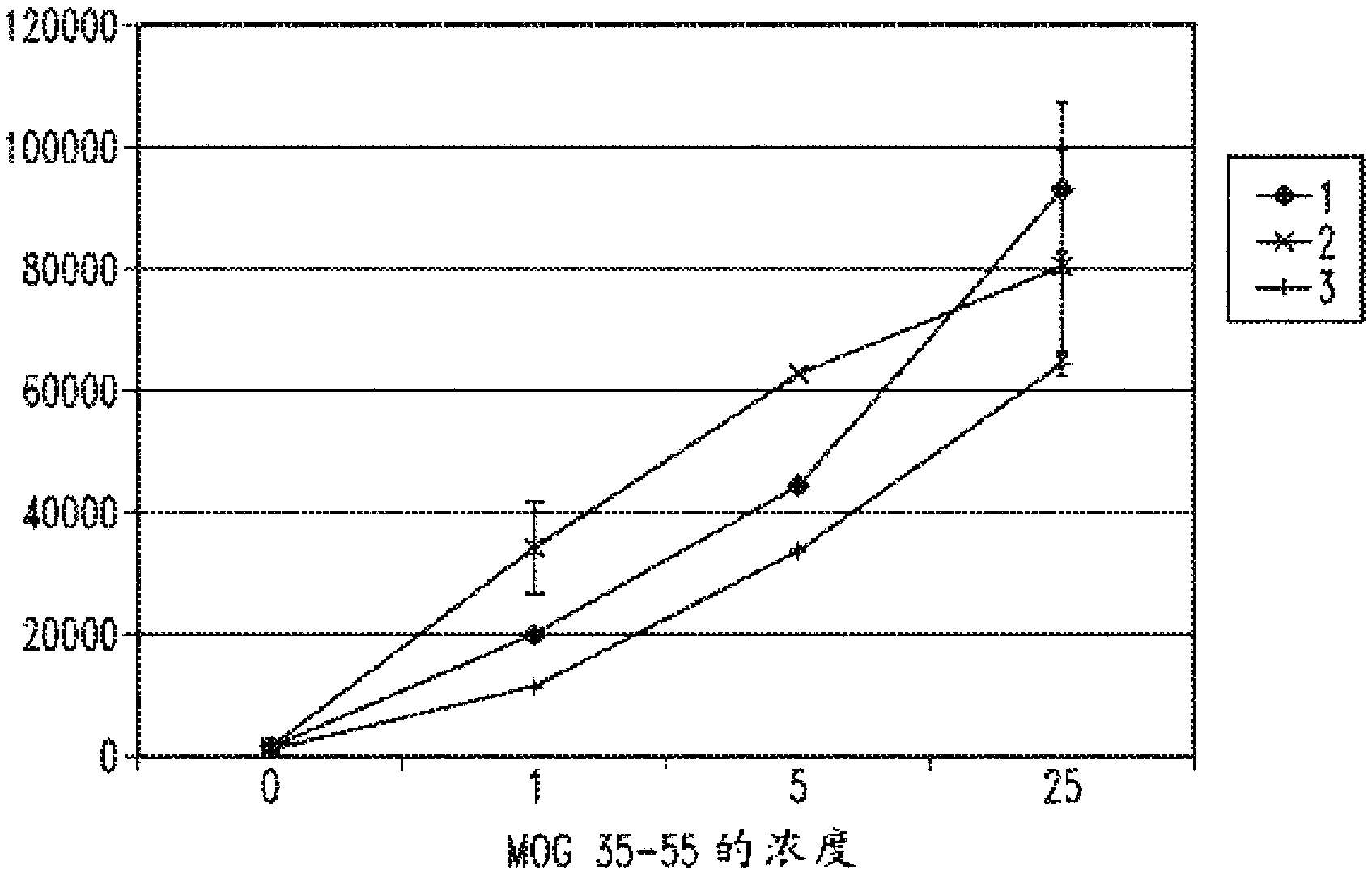
[0267] myelin oligodendrocyte glycoprotein (MOG)
[0268] Such as figure 2 As shown, lymphocyte proliferation in response to the MOG antigenic peptide was increased when cultured in the presence of the gas-enriched fluid of the present invention compared to pressurized oxygenated fluid (pressure tank) or deionized control fluid. Thus, the gas-enriched fluids of the present invention can amplify the proliferative response of lymphocytes to antigens of previously primed cells.
[0269] Myelin oligodendrocyte glycoprotein peptide 35-55 (MOG35-55) was synthesized corresponding to the known mouse sequence
[0270] (M-E-V-G-W-Y-R-S-P-F-S-R-O-V-H-L-Y-R-N-G-K) (SEQ ID NO: 1; see patent publication US20080139674, incorporated herein by reference, included for the purpose of this SEQ ID NO: 1). Next, place the 5x10 5 splenocytes were taken from MOG T cell receptor transgenic mice previously immunized with MOG, and reconstituted with the gas-enriched fluid of the present invention,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com